
How would you draw a Lewis structure for an atom that has an electronic configuration \[1{s^2}2{s^2}2{p^6}3{s^2}3{p^3}\]
Answer
572.1k+ views
Hint:In order to draw a Lewis dot structure for a given electronic configuration \[1{s^2}2{s^2}2{p^6}3{s^2}3{p^3}\], we must first find the atomic number of the atom. This can be done by counting the electrons present in the electronic configuration.
Complete answer:
Let us first understand what a Lewis dot structure is. It is the graphical representation of the electrons present around an atom. Let us move onto the problem. In order to draw a Lewis dot structure, we must first the chemical symbol of the element. This can be done only when we know the atomic number of the given element. For that we have to add the electrons from the electronic present. As we know that atomic number is the total number of electrons present in an atom. Therefore, the total electrons present is 15. The given element is Phosphorus, because 15 is the Atomic number of Phosphorus. It will be having five valence electrons in the outermost orbital.
Now let us write the Lewis dot structure for Phosphorus.
First write the chemical symbol of the given element. The number of valence electrons are written surrounding the phosphorus atom. The chemical dot structure for the Phosphorus is given below:
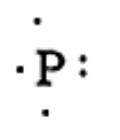
In this structure there are three single electrons and one pair of electrons. Therefore, Phosphorus can form three chemical bonds.
Note:
Lewis dot structure is very important because:
- It can predict geometry.
- Reactivity and polarity of inorganic and organic chemistry.
Complete answer:
Let us first understand what a Lewis dot structure is. It is the graphical representation of the electrons present around an atom. Let us move onto the problem. In order to draw a Lewis dot structure, we must first the chemical symbol of the element. This can be done only when we know the atomic number of the given element. For that we have to add the electrons from the electronic present. As we know that atomic number is the total number of electrons present in an atom. Therefore, the total electrons present is 15. The given element is Phosphorus, because 15 is the Atomic number of Phosphorus. It will be having five valence electrons in the outermost orbital.
Now let us write the Lewis dot structure for Phosphorus.
First write the chemical symbol of the given element. The number of valence electrons are written surrounding the phosphorus atom. The chemical dot structure for the Phosphorus is given below:
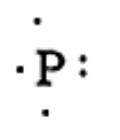
In this structure there are three single electrons and one pair of electrons. Therefore, Phosphorus can form three chemical bonds.
Note:
Lewis dot structure is very important because:
- It can predict geometry.
- Reactivity and polarity of inorganic and organic chemistry.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




