
Draw a concept map and explain classification of hydrocarbons based on carbon-carbon bonds with the help of examples.
Answer
573.3k+ views
Hint: We know that hydrocarbons as the name suggests, are the compound which will necessarily have hydrogen and carbon atoms, along with other atoms depending on the compound. This classification of hydrocarbons plays an aid in association with structural features with properties but does not require that a particular substance be assigned to a single class.
Complete step by step answer:
Hydrocarbons can be broadly categorised into two categories: open chain compounds and closed chain compounds. As the name suggests, under the classification of open chain compounds we will see the compounds which do not have ring structures, as in the carbon chain will be open from both the ends. Whereas in case of closed chain compounds, both the ends of the chain will be connected to each other, which results in formation of a ring. We will be discussing each of these categories in detail.
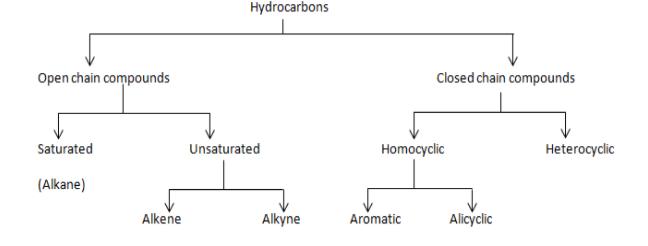
As we can see that the open chain is further divided into two groups, saturated and unsaturated hydrocarbons. Saturated, also called the alkanes are those hydrocarbons in which carbon atoms are attached to each other through single bonds only. Examples would include, methane, propane etc.
Whereas unsaturated hydrocarbons are those in which at least one carbon would have either double or triple bond with each other. Unsaturated hydrocarbons are further divided into two groups, alkenes and alkynes. Alkenes are those compounds which have at least one double bond across carbons, for instance propene, butene and alkynes are those in which the compound should have at least one triple bond, example propyne, butyne.
Closed chain compounds are further categorised into two groups, homocyclic and heterocyclic compounds. Homocyclic as the name suggests, are those in which the rings are made up of the same type of atom, as in the main ring will consist of only one type of atom, example cyclohexane, cyclopentane. And in heterocyclic compounds, there would be at least one atom which is different from the rest, example pyrrole, furan etc.
Homocyclic compounds are further categorised into two parts aromatic and acyclic.
Aromatic compounds are a type of chemical compound which consist of conjugated planar ring systems along with delocalized pi-electron clouds in place of individual alternating double and single bonds. Example benzene, and derivatives of benzene.
Alicyclic compounds are those which contain one or more all-carbon rings which may be either saturated or unsaturated, but it would not have aromatic character, example cyclohexane.
Note: Alkanes have the general formula \[{{C}_{n}}{{H}_{2n}}_{+2}\]. The general formula for the alkenes is \[{{C}_{n}}{{H}_{2n}}\] and that of alkyne is \[{{C}_{\mathbf{n}}}{{H}_{2n-2}}\].
Homocyclic compounds have a wide range of applications and the aromatic compounds have a characteristic smell.
Many hydrocarbons occur in nature. Along with making up fossil fuels, they are present in plants and trees, as, for example, in the form of pigments called carotenoids that occur in carrots and green leaves.
Complete step by step answer:
Hydrocarbons can be broadly categorised into two categories: open chain compounds and closed chain compounds. As the name suggests, under the classification of open chain compounds we will see the compounds which do not have ring structures, as in the carbon chain will be open from both the ends. Whereas in case of closed chain compounds, both the ends of the chain will be connected to each other, which results in formation of a ring. We will be discussing each of these categories in detail.

As we can see that the open chain is further divided into two groups, saturated and unsaturated hydrocarbons. Saturated, also called the alkanes are those hydrocarbons in which carbon atoms are attached to each other through single bonds only. Examples would include, methane, propane etc.
Whereas unsaturated hydrocarbons are those in which at least one carbon would have either double or triple bond with each other. Unsaturated hydrocarbons are further divided into two groups, alkenes and alkynes. Alkenes are those compounds which have at least one double bond across carbons, for instance propene, butene and alkynes are those in which the compound should have at least one triple bond, example propyne, butyne.
Closed chain compounds are further categorised into two groups, homocyclic and heterocyclic compounds. Homocyclic as the name suggests, are those in which the rings are made up of the same type of atom, as in the main ring will consist of only one type of atom, example cyclohexane, cyclopentane. And in heterocyclic compounds, there would be at least one atom which is different from the rest, example pyrrole, furan etc.
Homocyclic compounds are further categorised into two parts aromatic and acyclic.
Aromatic compounds are a type of chemical compound which consist of conjugated planar ring systems along with delocalized pi-electron clouds in place of individual alternating double and single bonds. Example benzene, and derivatives of benzene.
Alicyclic compounds are those which contain one or more all-carbon rings which may be either saturated or unsaturated, but it would not have aromatic character, example cyclohexane.
Note: Alkanes have the general formula \[{{C}_{n}}{{H}_{2n}}_{+2}\]. The general formula for the alkenes is \[{{C}_{n}}{{H}_{2n}}\] and that of alkyne is \[{{C}_{\mathbf{n}}}{{H}_{2n-2}}\].
Homocyclic compounds have a wide range of applications and the aromatic compounds have a characteristic smell.
Many hydrocarbons occur in nature. Along with making up fossil fuels, they are present in plants and trees, as, for example, in the form of pigments called carotenoids that occur in carrots and green leaves.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




