
How do you draw a (\[2R\], \[3R\])\[ - 2,3 - dichloropentane\]?
Answer
554.4k+ views
Hint: Both \[Cl\] groups ought to point in reverse. In the wake of relegating needs, the rotation of this will be like counter-clock wise (which implies \[S\]) anyway in light of the fact that the most priority group is pointing in reverse, the \[S\] becomes \[R\].
Complete step by step answer:
The structure is beneath...
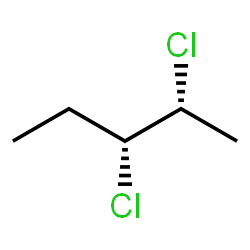
- \[Molecular{\text{ }}Formula \to {C_5}{H_{10}}C{l_2}\]
- \[Average{\text{ }}mass \to 141.039Da\]
- \[Monoisotopic{\text{ }}mass \to 140.015961{\text{ }}Da\]
Draw the structure of (\[2R\], \[3R\])\[ - 2,3 - dichloropentane\]. Utilize strong or hashed wedges to demonstrate the setup at uneven \[C\] atoms. Draw the molecule on the image by selection fasteners from the Tools (for bonds), Atoms, and Innovative Template toolbars. The single bond is dynamic as a matter of course. Show the suitable stereochemistry by picking the ruined or obstructed buttons and afterward clicking a bond on the canvas.
The guidelines have developed to cover numerous circumstances; however, the essential principles are:
Think about the main atom of each piece of the molecule. An atom with higher atomic number has higher need. (for example, \[I{\text{ }} > {\text{ }}Cl{\text{ }} > {\text{ }}C{\text{ }} > {\text{ }}H\]). On the off chance that the principal atom of two groups is the equivalent, consider the second atom(s) similarly as the first. (for example, \[ - C{\left( {C{H_3}} \right)_3}\; > {\text{ }} - CH{\left( {C{H_3}} \right)_2}\; > {\text{ }} - C{H_2}C{H_3}\; > {\text{ }} - C{H_3}\]). On the off chance that this doesn't allocate need, consider the following atoms until there is a distinction.
\[R - \] and \[S - \] documentation utilize the CIP need rules for the task of the supreme design around a stereocenter. To start with, appointment needs as portrayed above to each bonded bunch encompassing the stereocenter (\[1\], most noteworthy to\[4\], least). Second, point the least needed (\[4\]) atom away from you. Follow the heading of the leftover \[3\] needs from most elevated to least needed (most reduced to most noteworthy number, \[1 < 2 < 3\]).
Note:
Stereoisomers are appropriately named utilizing the Cahn-Ingold-Prelog (CIP) need rules to choose which parts of the molecule to think about first. A counterclockwise bearing is a \[S\] (sinister, Latin for left) arrangement. A clockwise heading is a \[R\] (rectus, Latin for right) setup.
Complete step by step answer:
The structure is beneath...

- \[Molecular{\text{ }}Formula \to {C_5}{H_{10}}C{l_2}\]
- \[Average{\text{ }}mass \to 141.039Da\]
- \[Monoisotopic{\text{ }}mass \to 140.015961{\text{ }}Da\]
Draw the structure of (\[2R\], \[3R\])\[ - 2,3 - dichloropentane\]. Utilize strong or hashed wedges to demonstrate the setup at uneven \[C\] atoms. Draw the molecule on the image by selection fasteners from the Tools (for bonds), Atoms, and Innovative Template toolbars. The single bond is dynamic as a matter of course. Show the suitable stereochemistry by picking the ruined or obstructed buttons and afterward clicking a bond on the canvas.
The guidelines have developed to cover numerous circumstances; however, the essential principles are:
Think about the main atom of each piece of the molecule. An atom with higher atomic number has higher need. (for example, \[I{\text{ }} > {\text{ }}Cl{\text{ }} > {\text{ }}C{\text{ }} > {\text{ }}H\]). On the off chance that the principal atom of two groups is the equivalent, consider the second atom(s) similarly as the first. (for example, \[ - C{\left( {C{H_3}} \right)_3}\; > {\text{ }} - CH{\left( {C{H_3}} \right)_2}\; > {\text{ }} - C{H_2}C{H_3}\; > {\text{ }} - C{H_3}\]). On the off chance that this doesn't allocate need, consider the following atoms until there is a distinction.
\[R - \] and \[S - \] documentation utilize the CIP need rules for the task of the supreme design around a stereocenter. To start with, appointment needs as portrayed above to each bonded bunch encompassing the stereocenter (\[1\], most noteworthy to\[4\], least). Second, point the least needed (\[4\]) atom away from you. Follow the heading of the leftover \[3\] needs from most elevated to least needed (most reduced to most noteworthy number, \[1 < 2 < 3\]).
Note:
Stereoisomers are appropriately named utilizing the Cahn-Ingold-Prelog (CIP) need rules to choose which parts of the molecule to think about first. A counterclockwise bearing is a \[S\] (sinister, Latin for left) arrangement. A clockwise heading is a \[R\] (rectus, Latin for right) setup.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




