
Double bond equivalent of the following is:
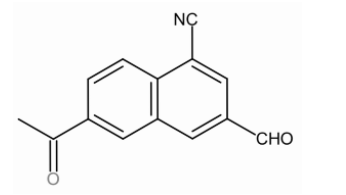
A. 7
B. 11
C. 6
D. None of these

Answer
600.3k+ views
Hint: The double bond equivalent or level of unsaturation is the number of unsaturation present in an organic molecule. We can find this by the formula:
\[DBE=C+\dfrac{H}{2}-\dfrac{X}{2}+\dfrac{N}{2}\]
Complete step by step solution:
The term unsaturation here means a double bond or a ring system. For example, in benzene there are 3 double bonds and 1 ring which gives us 4 DBE.
Moreover, a triple bond can be regarded as DBE=2.
It must be noted that in the formula
\[DBE=C+\dfrac{H}{2}-\dfrac{X}{2}+\dfrac{N}{2}\]
Presence of an oxygen atom does not affect the DBE calculation.
Now, in this compound the number of Carbon atoms are 14.
Therefore, from the above solution we can conclude that the correct answer is (b).
Note: Remember, the degrees of unsaturation only give the sum of double bonds, triple bonds and/or rings. For instance, a degree of unsaturation of 3 can contain 3 rings, 2 rings and one double bond, 1 ring and two double bonds, 1 ring and triple bond, 1 double bond and one triple bond, or 3 double bonds.
\[DBE=C+\dfrac{H}{2}-\dfrac{X}{2}+\dfrac{N}{2}\]
Complete step by step solution:
The term unsaturation here means a double bond or a ring system. For example, in benzene there are 3 double bonds and 1 ring which gives us 4 DBE.
Moreover, a triple bond can be regarded as DBE=2.
It must be noted that in the formula
\[DBE=C+\dfrac{H}{2}-\dfrac{X}{2}+\dfrac{N}{2}\]
where, X is the total number of halogens, namely Cl, Br, F and I present in the structure.
C = number of carbon atoms.
H = number of Hydrogen atoms.
N = number of nitrogen atoms.
Presence of an oxygen atom does not affect the DBE calculation.
Now, in this compound the number of Carbon atoms are 14.
Number of Hydrogen atoms is 9.
Number of Nitrogen atoms is 1.
Number of halogen atoms is 0
On applying the DBE formula:
\[=14+1-\dfrac{9}{2}+\dfrac{1}{2}\]
= 15 - 4
= 11
Therefore, from the above solution we can conclude that the correct answer is (b).
Note: Remember, the degrees of unsaturation only give the sum of double bonds, triple bonds and/or rings. For instance, a degree of unsaturation of 3 can contain 3 rings, 2 rings and one double bond, 1 ring and two double bonds, 1 ring and triple bond, 1 double bond and one triple bond, or 3 double bonds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




