
How does the activation energy affect reaction rate?
Answer
550.5k+ views
Hint: The reaction continues at a speed. On the off chance that the activation energy is high, a small amount of successful crashes is little, and the reaction happens gradually. We realize that in an endothermic reaction, the energy delivered is positive. \[\therefore \Delta H{\text{ }} = {\text{ }} + {\text{ }}ve\](enthalpy of a synthetic reaction is positive)
Complete step by step answer:
The activation energy of a synthetic reaction is somewhat similar to that "bump" you need to get over to get yourself up. Indeed, even energy-delivering (exergonic) reactions require some measure of energy contribution to get moving, before they can continue with their energy-delivering steps. This underlying energy input, which is later repaid as the reaction continues, is known as the enactment energy and is abridged\[{E_A}\].
The change state is a high-energy state, and some measure of energy – the activation energy – should be included in the request for the atom to arrive at it. Since the change state is precarious, reactant particles don't remain there long, yet rapidly continue to the following stage of the compound reaction.In general, the progress condition of a reaction is consistently at a higher energy level than the reactants or items, with the end goal that \[{E_A}\] consistently has a positive worth – free of whether the reaction is endergonic or exergonic by and large. The activation energy appeared in the outline beneath is for the forward reaction (reactants → items), which is exergonic. In the event that the reaction were to continue the opposite way (endergonic), the change state would continue as before, yet the activation energy would be bigger. This is on the grounds that the item atoms are lower-energy and would along these lines need more energy added to arrive at the progress state at the highest point of the reaction "slope." (An enactment energy bolt for the opposite reaction would stretch out from the items up to the change state.)
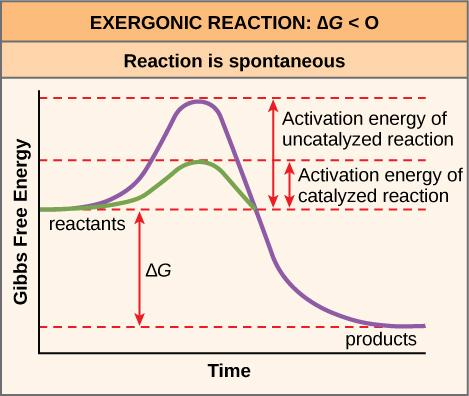
Reaction organizes charts for an exergonic reaction. Albeit the items are at a lower energy level than the reactants (free energy is delivered in going from reactants to items), there is as yet a "bump" in the vigorous way of the reaction, mirroring the development of the high-energy change state. The activation energy for the forward reaction is the measure of free energy that should be added to go from the energy level of the reactants to the energy level of the change state.
The wellspring of activation energy is regularly heat, with reactant atoms retaining nuclear power from their environmental factors. This nuclear power speeds up the movement of the reactant particles, expanding the recurrence and power of their crashes, and furthermore shakes the iotas and bonds inside the individual atoms, making it more probable that bonds will break. When a reactant particle retains enough energy to arrive at the change state, it can continue through the rest of the reaction.
The activation energy of a substance reaction is firmly identified with its rate. In particular, the higher the activation energy, the more slow the compound reaction will be. This is on the grounds that atoms can just finish the reaction whenever they have arrived at the highest point of the enactment energy obstruction. The higher the hindrance is, the less particles that will have enough energy to make it over out of nowhere.
Numerous reactions have such high enactment energies that they fundamentally don't continue at all without a contribution of energy. For example, the ignition of a fuel like propane discharges energy, however the pace of reaction is adequately zero at room temperature. (Honestly, this is something to be thankful for – it wouldn't be so incredible if propane canisters unexpectedly combusted on the rack!) Once a sparkle has given enough energy to get a few atoms over the enactment energy hindrance, those particles complete the reaction, delivering energy. The delivered energy helps other fuel particles get over the energy hindrance too, prompting a chain reaction.
Note: Most compound reactions that occur in cells resemble the hydrocarbon burning model: the enactment energy is excessively high for the reactions to continue altogether at surrounding temperature. From the outset, this appears to be an issue; all things considered, you can't set off a flash within a cell without causing harm. Luckily, it's conceivable to bring down the activation energy of a reaction, and to accordingly build reaction rate. The way toward accelerating a reaction by decreasing its activation energy is known as catalysis, and the factor that is added to bring down the enactment energy is known as an impetus.
Complete step by step answer:
The activation energy of a synthetic reaction is somewhat similar to that "bump" you need to get over to get yourself up. Indeed, even energy-delivering (exergonic) reactions require some measure of energy contribution to get moving, before they can continue with their energy-delivering steps. This underlying energy input, which is later repaid as the reaction continues, is known as the enactment energy and is abridged\[{E_A}\].
The change state is a high-energy state, and some measure of energy – the activation energy – should be included in the request for the atom to arrive at it. Since the change state is precarious, reactant particles don't remain there long, yet rapidly continue to the following stage of the compound reaction.In general, the progress condition of a reaction is consistently at a higher energy level than the reactants or items, with the end goal that \[{E_A}\] consistently has a positive worth – free of whether the reaction is endergonic or exergonic by and large. The activation energy appeared in the outline beneath is for the forward reaction (reactants → items), which is exergonic. In the event that the reaction were to continue the opposite way (endergonic), the change state would continue as before, yet the activation energy would be bigger. This is on the grounds that the item atoms are lower-energy and would along these lines need more energy added to arrive at the progress state at the highest point of the reaction "slope." (An enactment energy bolt for the opposite reaction would stretch out from the items up to the change state.)

Reaction organizes charts for an exergonic reaction. Albeit the items are at a lower energy level than the reactants (free energy is delivered in going from reactants to items), there is as yet a "bump" in the vigorous way of the reaction, mirroring the development of the high-energy change state. The activation energy for the forward reaction is the measure of free energy that should be added to go from the energy level of the reactants to the energy level of the change state.
The wellspring of activation energy is regularly heat, with reactant atoms retaining nuclear power from their environmental factors. This nuclear power speeds up the movement of the reactant particles, expanding the recurrence and power of their crashes, and furthermore shakes the iotas and bonds inside the individual atoms, making it more probable that bonds will break. When a reactant particle retains enough energy to arrive at the change state, it can continue through the rest of the reaction.
The activation energy of a substance reaction is firmly identified with its rate. In particular, the higher the activation energy, the more slow the compound reaction will be. This is on the grounds that atoms can just finish the reaction whenever they have arrived at the highest point of the enactment energy obstruction. The higher the hindrance is, the less particles that will have enough energy to make it over out of nowhere.
Numerous reactions have such high enactment energies that they fundamentally don't continue at all without a contribution of energy. For example, the ignition of a fuel like propane discharges energy, however the pace of reaction is adequately zero at room temperature. (Honestly, this is something to be thankful for – it wouldn't be so incredible if propane canisters unexpectedly combusted on the rack!) Once a sparkle has given enough energy to get a few atoms over the enactment energy hindrance, those particles complete the reaction, delivering energy. The delivered energy helps other fuel particles get over the energy hindrance too, prompting a chain reaction.
Note: Most compound reactions that occur in cells resemble the hydrocarbon burning model: the enactment energy is excessively high for the reactions to continue altogether at surrounding temperature. From the outset, this appears to be an issue; all things considered, you can't set off a flash within a cell without causing harm. Luckily, it's conceivable to bring down the activation energy of a reaction, and to accordingly build reaction rate. The way toward accelerating a reaction by decreasing its activation energy is known as catalysis, and the factor that is added to bring down the enactment energy is known as an impetus.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




