
Why does boron trifluoride behave as a Lewis acid?
Answer
583.2k+ views
Hint: In the early 1920s, a scientist named G. N. Lewis suggested a theory named as the acid – base theory which defined the chemical characteristic of acids and bases. According to this theory, acids and bases are classified according to their ability to share electrons. The general conception of acids being classified as compounds containing only \[{H^ + }\] ions and bases containing only \[O{H^ - }\] ions was changed by this theory.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
Lewis acids can be defined as chemical species which are capable of accepting electrons. This is possible only if there are sufficient voids or empty species in the valence shells of the given chemical species. In more simpler terms, Lewis acids can be explained as chemical species which have sufficient empty spaces to accept lone pairs of electrons, which would in turn satisfy their octet.
On the other hand, Lewis bases are complete opposite compounds as compared to Lewis acids. Lewis bases can be identified as chemical species which are capable of donating lone pairs of electrons. To fulfil this condition, Lewis bases must have excess electron pairs in their valence shells. Now, excess electrons do not mean electron pairs exist in abundance to the actual electronic configuration of the molecule. What it actually means is that the valence shell has just enough electrons that prevent it from being half filled. This would motivate the cause for electron donation to achieve better stability.
The molecular formula of boron trifluoride is \[B{F_3}\] and its molecular structure can be given as:
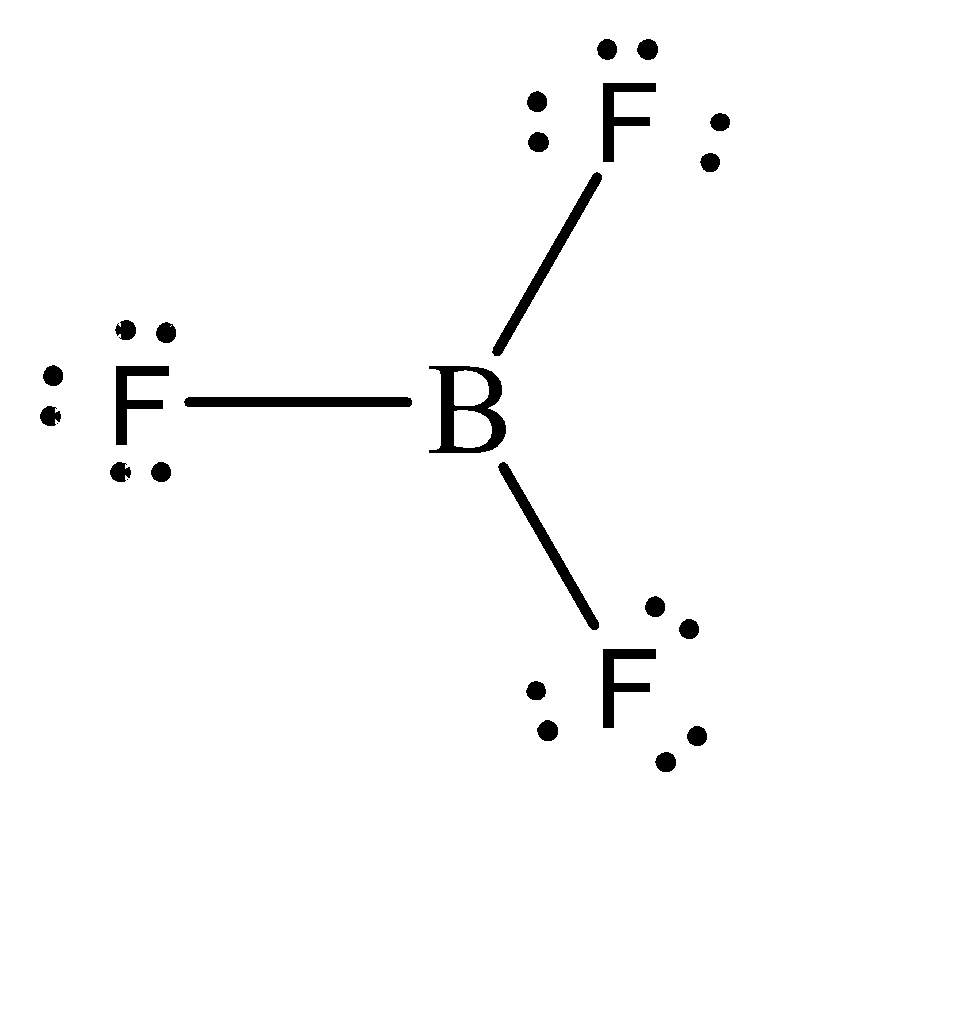
We can observe that Boron forms 3 sigma bonds with 3 fluorine atoms. Hence, the total number of electrons in the valence shell of Boron is 6. This means that it is short of 2 electrons to complete its octet. Hence, this compound would tend to accept 2 electrons to complete the octet. Because of this, boron trifluoride can be considered as a Lewis Acid.
Note: The most stable state is to leave the boron with an empty p orbital. \[B{F_3}\] is a planar molecule because it does not have a lone pair, which makes it have a trigonal planar geometry. \[B{F_3}\] is considered a Lewis acid because it accepts electrons at its empty p orbital.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
Lewis acids can be defined as chemical species which are capable of accepting electrons. This is possible only if there are sufficient voids or empty species in the valence shells of the given chemical species. In more simpler terms, Lewis acids can be explained as chemical species which have sufficient empty spaces to accept lone pairs of electrons, which would in turn satisfy their octet.
On the other hand, Lewis bases are complete opposite compounds as compared to Lewis acids. Lewis bases can be identified as chemical species which are capable of donating lone pairs of electrons. To fulfil this condition, Lewis bases must have excess electron pairs in their valence shells. Now, excess electrons do not mean electron pairs exist in abundance to the actual electronic configuration of the molecule. What it actually means is that the valence shell has just enough electrons that prevent it from being half filled. This would motivate the cause for electron donation to achieve better stability.
The molecular formula of boron trifluoride is \[B{F_3}\] and its molecular structure can be given as:

We can observe that Boron forms 3 sigma bonds with 3 fluorine atoms. Hence, the total number of electrons in the valence shell of Boron is 6. This means that it is short of 2 electrons to complete its octet. Hence, this compound would tend to accept 2 electrons to complete the octet. Because of this, boron trifluoride can be considered as a Lewis Acid.
Note: The most stable state is to leave the boron with an empty p orbital. \[B{F_3}\] is a planar molecule because it does not have a lone pair, which makes it have a trigonal planar geometry. \[B{F_3}\] is considered a Lewis acid because it accepts electrons at its empty p orbital.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




