
Do the following conversion in not more than two steps:
Ethylbenzene to benzoic acid
Answer
497.7k+ views
Hint: We are given the chemical names of the reactant and product. In order to convert one compound to another, we first need to know their structures and then choose the appropriate reagent for this conversion. Reagents are the chemical compounds which are used in different chemical reactions to make the reaction proceed in the desired direction.
Complete Step By Step Answer:
Reagents are the chemical compounds which are used in the chemical conversions and many other chemical reactions. Each reagent has a different role to play in a chemical reaction.
Now, we will look at the structures of both the reactant (ethyl benzene) and product (benzoic acid).
The structure of ethyl benzene is:
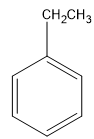
The structure of benzoic acid:
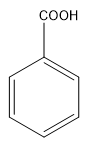
So, in the above conversion, we have to convert ethyl group into carboxylic acid group. This means that oxidation is taking place in the above reaction. Now, the oxidising agent (reagent) used for this conversion is alkaline $ KMn{O_4} $ (Alkaline Potassium permanganate).
Therefore, the reaction to convert ethylbenzene to benzoic acid is given as follows:
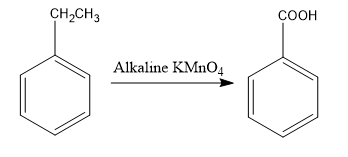
Note:
We should note that there are many oxidising agents but they have different roles to play in a chemical reaction. For example chromium oxide is a mild oxidising agent which can convert primary alcohol to aldehyde group and secondary alcohol to a ketone. While a strong oxidising agent like alkaline potassium permanganate converts primary alcohol to the carboxylic acid group.
Complete Step By Step Answer:
Reagents are the chemical compounds which are used in the chemical conversions and many other chemical reactions. Each reagent has a different role to play in a chemical reaction.
Now, we will look at the structures of both the reactant (ethyl benzene) and product (benzoic acid).
The structure of ethyl benzene is:

The structure of benzoic acid:

So, in the above conversion, we have to convert ethyl group into carboxylic acid group. This means that oxidation is taking place in the above reaction. Now, the oxidising agent (reagent) used for this conversion is alkaline $ KMn{O_4} $ (Alkaline Potassium permanganate).
Therefore, the reaction to convert ethylbenzene to benzoic acid is given as follows:

Note:
We should note that there are many oxidising agents but they have different roles to play in a chemical reaction. For example chromium oxide is a mild oxidising agent which can convert primary alcohol to aldehyde group and secondary alcohol to a ketone. While a strong oxidising agent like alkaline potassium permanganate converts primary alcohol to the carboxylic acid group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




