
How could you distinguish between the following pair of the compounds from their IR spectra? \[OHC{H_2}C{H_2}CHO\] (3-hydroxypropanal) and \[C{H_3}C{H_2}COOH\] (propanoic acid)
Answer
550.5k+ views
Hint: IR spectroscopy (Infrared spectroscopy / vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used for study and identification of chemical substances or the functional groups in solid, liquid, or gaseous forms. The method of IR spectroscopy is conducted with an instrument called an IR spectrometer/ spectrophotometer which produces an IR spectrum.
Complete step-by-step answer:
IR spectrum can be visualized in a graph of infrared light absorbance/ transmittance on the vertical axis and frequency/ wavelength on the horizontal axis. Typical units of frequency used in IR spectra are reciprocal centimetres, shown as \[c{m^{ - 1}}\] . Units of IR wavelength are commonly given in micrometres, shown as \[\;\mu m\] , which are reciprocally related to wavenumbers.
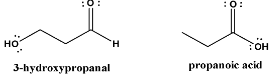
If we notice the functional groups, they are different;
The 3-hydroxypropanal compound has an alcohol \[ - OH\] and an aldehyde \[ - C = O\] group, while the propanoic acid compound has a carboxyl hydroxyl \[ - OH\] group and a carboxyl carbonyl \[ - C = O\] group.
The main differences between these two molecules in the IR spectra are in the \[ - OH\] stretches and in the \[ - C = O\] stretches.
While the alcohol \[ - OH\] stretch is broader, the carboxylic \[ - OH\] stretch is less broad.
On the other hand, the aldehyde \[ - C = O\] stretch is sharp and "well-defined" and the carboxylic \[ - C = O\] stretch is broader and more "smeared".
Note:The significant vibrational motion: In 3-hydroxypropanal –
\[C = O\] stretch (aldehyde) -- \[1740-1720\,c{m^{ - 1}}\] (saturated) and \[1715-1680{\text{ }}c{m^{ - 1}}\] (conjugated); medium; sharp
\[O - H\] stretch (alcohol) -- \[3650-3550\,c{m^{ - 1}}\] and \[3550-3200{\text{ }}c{m^{ - 1}}\] ; medium to strong; broad
The significant vibrational motion: In propanoic acid –
\[C = O\] stretch (carboxylic acid) -- \[1725-1700\,c{m^{ - 1}}\] (saturated) and \[1715-1680{\text{ }}c{m^{ - 1}}\] (conjugated); strong; sharp
\[O - H\] stretch (carboxylic acid) -- \[3200-2500{\text{ }}c{m^{ - 1}}\] ; medium to weak; broad
Complete step-by-step answer:
IR spectrum can be visualized in a graph of infrared light absorbance/ transmittance on the vertical axis and frequency/ wavelength on the horizontal axis. Typical units of frequency used in IR spectra are reciprocal centimetres, shown as \[c{m^{ - 1}}\] . Units of IR wavelength are commonly given in micrometres, shown as \[\;\mu m\] , which are reciprocally related to wavenumbers.

If we notice the functional groups, they are different;
The 3-hydroxypropanal compound has an alcohol \[ - OH\] and an aldehyde \[ - C = O\] group, while the propanoic acid compound has a carboxyl hydroxyl \[ - OH\] group and a carboxyl carbonyl \[ - C = O\] group.
The main differences between these two molecules in the IR spectra are in the \[ - OH\] stretches and in the \[ - C = O\] stretches.
While the alcohol \[ - OH\] stretch is broader, the carboxylic \[ - OH\] stretch is less broad.
On the other hand, the aldehyde \[ - C = O\] stretch is sharp and "well-defined" and the carboxylic \[ - C = O\] stretch is broader and more "smeared".
Note:The significant vibrational motion: In 3-hydroxypropanal –
\[C = O\] stretch (aldehyde) -- \[1740-1720\,c{m^{ - 1}}\] (saturated) and \[1715-1680{\text{ }}c{m^{ - 1}}\] (conjugated); medium; sharp
\[O - H\] stretch (alcohol) -- \[3650-3550\,c{m^{ - 1}}\] and \[3550-3200{\text{ }}c{m^{ - 1}}\] ; medium to strong; broad
The significant vibrational motion: In propanoic acid –
\[C = O\] stretch (carboxylic acid) -- \[1725-1700\,c{m^{ - 1}}\] (saturated) and \[1715-1680{\text{ }}c{m^{ - 1}}\] (conjugated); strong; sharp
\[O - H\] stretch (carboxylic acid) -- \[3200-2500{\text{ }}c{m^{ - 1}}\] ; medium to weak; broad
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




