
Distinguish between phenol and benzoic acid with a suitable chemical equation.
Answer
524.2k+ views
Hint: The concept of organic chemistry that deals with the identification of the compounds by the tests which includes the treatment of these two compounds with ferric chloride and the one which forms the complex gives the correct answer. Write the reaction based on this fact.
Complete answer:
We have studied about various tests used for the detection and identification of the given compounds in the laboratories.
We shall now see the tests used for the differentiation of phenol and benzoic acid.
- To test for the carboxylic acid functional group, we have known well that the sodium bicarbonate test gives the positive test.
Thus, now if we treat benzoic acid with sodium bicarbonate, we get sodium benzoate and evolution of carbon dioxide with brick efflorescence that confirms the presence of carboxylic acid group. The reaction is shown below,
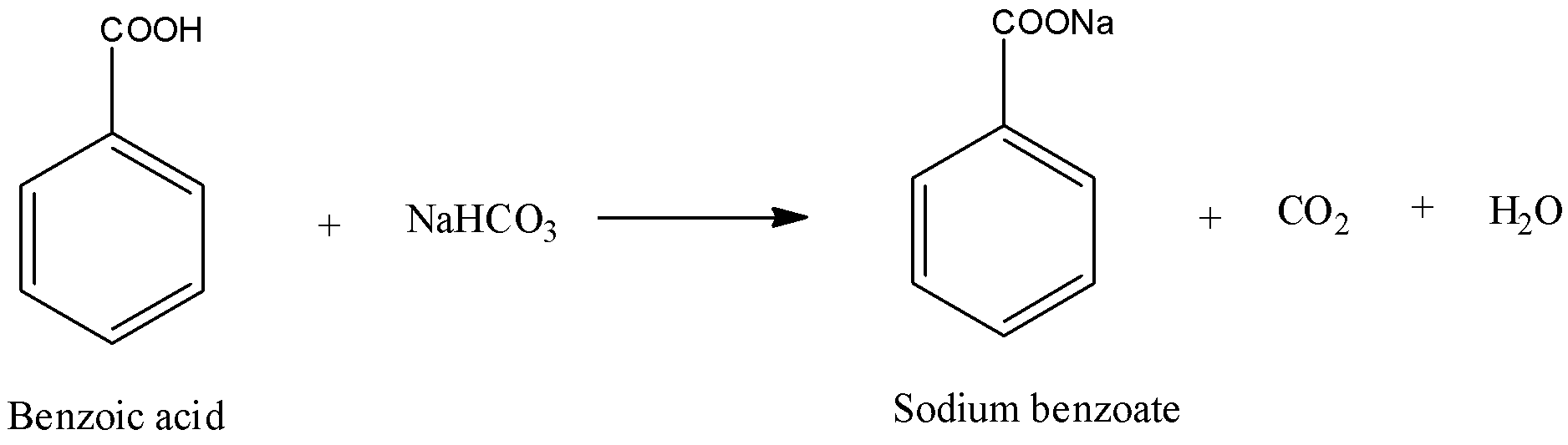
Now, if we subject phenol to this test then there is no reaction and therefore, this distinguishes phenol from carboxylic acid.
Now, another test is the ferric chloride test and if we treat phenol with ferric chloride, it gives a positive test for phenols and it confirms its presence. During this reaction, the product formed will have either red, green, blue or purple that indicates the presence of phenol ring. The reaction is as shown,
$3{{C}_{6}}{{H}_{5}}OH+FeC{{l}_{3}}\to {{({{C}_{6}}{{H}_{5}}O)}_{3}}Fe+3HCl$
But, if we treat benzoic acid with this reagent, then there is no much reaction.
Therefore, the correct answer is phenol and benzoic acid can be differentiated by sodium bicarbonate test or by ferric chloride test.
Note:
Note that phenol usually gives violet colouration with the neutral ferric chloride solution and also benzoic acid gives buff coloured that is pale dull yellow coloured precipitate with neutral ferric chloride solution.
Complete answer:
We have studied about various tests used for the detection and identification of the given compounds in the laboratories.
We shall now see the tests used for the differentiation of phenol and benzoic acid.
- To test for the carboxylic acid functional group, we have known well that the sodium bicarbonate test gives the positive test.
Thus, now if we treat benzoic acid with sodium bicarbonate, we get sodium benzoate and evolution of carbon dioxide with brick efflorescence that confirms the presence of carboxylic acid group. The reaction is shown below,

Now, if we subject phenol to this test then there is no reaction and therefore, this distinguishes phenol from carboxylic acid.
Now, another test is the ferric chloride test and if we treat phenol with ferric chloride, it gives a positive test for phenols and it confirms its presence. During this reaction, the product formed will have either red, green, blue or purple that indicates the presence of phenol ring. The reaction is as shown,
$3{{C}_{6}}{{H}_{5}}OH+FeC{{l}_{3}}\to {{({{C}_{6}}{{H}_{5}}O)}_{3}}Fe+3HCl$
But, if we treat benzoic acid with this reagent, then there is no much reaction.
Therefore, the correct answer is phenol and benzoic acid can be differentiated by sodium bicarbonate test or by ferric chloride test.
Note:
Note that phenol usually gives violet colouration with the neutral ferric chloride solution and also benzoic acid gives buff coloured that is pale dull yellow coloured precipitate with neutral ferric chloride solution.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




