
When dimethylglyoxime solution is added to an aqueous solution of nickel (II) chloride followed by ammonium hydroxide, then which of the following statements are incorrect?
This question has multiple correct questions
(a) No precipitate is obtained
(b) A blue colored ppt. is obtained
(c) rosy red colored ppt. is obtained
(d) A black colored ppt. is obtained
Answer
560.7k+ views
Hint Dimethyl glyoxime is a chelate i.e. it donates the electrons to the central metal atom through the two donor atoms and when this is made to undergoes reaction with the aqueous solution of nickel, it results into the formation of chelate which undergoes precipitation by ammonium hydroxide and colored ppt. are obtained. Now solve it.
Complete answer:
First of all, let’s discuss what dimethylglyoxime is. Dimethylglyoxime is the chelating ligands and are those ligands (those molecules which are attached to the central metal atom through the coordinate bonds) which binds the central metal atom through two or more donor atoms of the ligands and results in the formation of cyclic ring compounds known as chelates and the process is known as chelation.
Chelating ligands are actually multidentate ligands which upon coordination with the central metal atom results in the formation of cyclic(ring) compounds. All the types of bidentate, tridentate, tetradentate, pentadentate and hexadentate ligands are known to form a variety of chelates.
Dimethylglyoxime, it is a bidentate ligand in which both the nitrogen atoms of a dmg molecule donates electrons to the central metal atom and forms the coordinate bonds. And thus, is a chelating ligand and forms the chelates.
Now considering the statement;
When dimethylglyoxime solution is added to an aqueous solution of nickel II chloride , it results in the formation of chelate i.e. the bis( dimethylglyoximato) nickel(II) which gives rosy red precipitates. The reaction occurs as;
In DMG i.e. Dimethylglyoxime, it is a bidentate ligand in which both the nitrogen atoms of a dmg molecule donates electrons to the central metal atom and forms the coordinate bonds. And thus, is a chelating ligand and forms the chelates. DMG is mostly used for the estimation of nickel as follows:
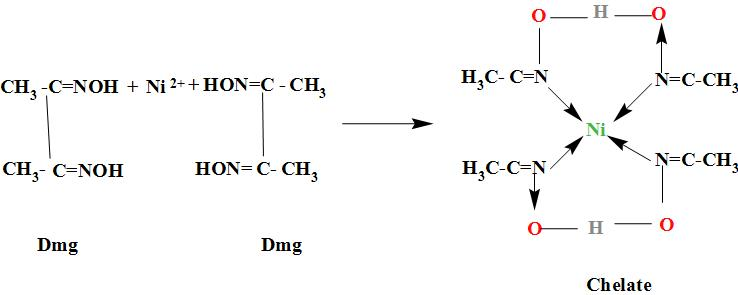
So, when dimethylglyoxime solution is added to an aqueous solution of nickel II chloride followed by ammonium hydroxide, rosy red colored ppts are obtained.
So, hence option (a) , (b) and (d) are correct.
Note: Chelates or the chelated complexes have been found to be much more stable than the similar complexes with unidentate ligand (uni means one and dentate refers to the denticity which means the number the donor groups which can donate electron to the central metal atom and forms the coordinate bond with it). It is so because the chelate, as we know, is a cyclic ring and involves the breaking of the two bonds unlike the one in non- chelated complexes.
Complete answer:
First of all, let’s discuss what dimethylglyoxime is. Dimethylglyoxime is the chelating ligands and are those ligands (those molecules which are attached to the central metal atom through the coordinate bonds) which binds the central metal atom through two or more donor atoms of the ligands and results in the formation of cyclic ring compounds known as chelates and the process is known as chelation.
Chelating ligands are actually multidentate ligands which upon coordination with the central metal atom results in the formation of cyclic(ring) compounds. All the types of bidentate, tridentate, tetradentate, pentadentate and hexadentate ligands are known to form a variety of chelates.
Dimethylglyoxime, it is a bidentate ligand in which both the nitrogen atoms of a dmg molecule donates electrons to the central metal atom and forms the coordinate bonds. And thus, is a chelating ligand and forms the chelates.
Now considering the statement;
When dimethylglyoxime solution is added to an aqueous solution of nickel II chloride , it results in the formation of chelate i.e. the bis( dimethylglyoximato) nickel(II) which gives rosy red precipitates. The reaction occurs as;
In DMG i.e. Dimethylglyoxime, it is a bidentate ligand in which both the nitrogen atoms of a dmg molecule donates electrons to the central metal atom and forms the coordinate bonds. And thus, is a chelating ligand and forms the chelates. DMG is mostly used for the estimation of nickel as follows:

So, when dimethylglyoxime solution is added to an aqueous solution of nickel II chloride followed by ammonium hydroxide, rosy red colored ppts are obtained.
So, hence option (a) , (b) and (d) are correct.
Note: Chelates or the chelated complexes have been found to be much more stable than the similar complexes with unidentate ligand (uni means one and dentate refers to the denticity which means the number the donor groups which can donate electron to the central metal atom and forms the coordinate bond with it). It is so because the chelate, as we know, is a cyclic ring and involves the breaking of the two bonds unlike the one in non- chelated complexes.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




