
Determine the empirical formula of an oxide of iron which has 69.9% iron and 30.1% dioxygen by mass.
Answer
525.2k+ views
Hint: The empirical formula of a chemical compound is calculated with the help of two factors i.e., the percentage composition of elements and atomic mass of the element.
Complete step by step answer:
The empirical formula is the chemical formula that tells the simplest whole-number ratio of the atoms of all elements present in one molecule.
It is deduced by- (a) percentage composition of elements (b)- atomic masses.
There are some steps for calculating the empirical formula:
i)- To convert the mass percent into grams. The given mass percentage represents the masses of the element in grams.
ii)- To calculate the number of moles. Divide the percentage of each element by its atomic mass.
\[\text{moles of the element= }\frac{\text{mass of the element}}{\text{At}\text{. mass of the element}}\]
iii)-To calculate the simplest molar ration. Divide the moles obtained in step (i) by the smallest quotient or the least value from amongst the values obtained for each element. This gives the simplest molar ratio.
iv)- To calculate the simplest whole number ratio. The simplest molar ratios, as calculated in step 2, are generally whole numbers. If not, then (a) raise the values to the nearest whole number, or (b) multiply the simplest atomic ratio by a suitable integer.
v)- To write the empirical formula. Insert the numerical value of the simplest whole number.
According to the following steps, for iron and oxygen is solved in the table:
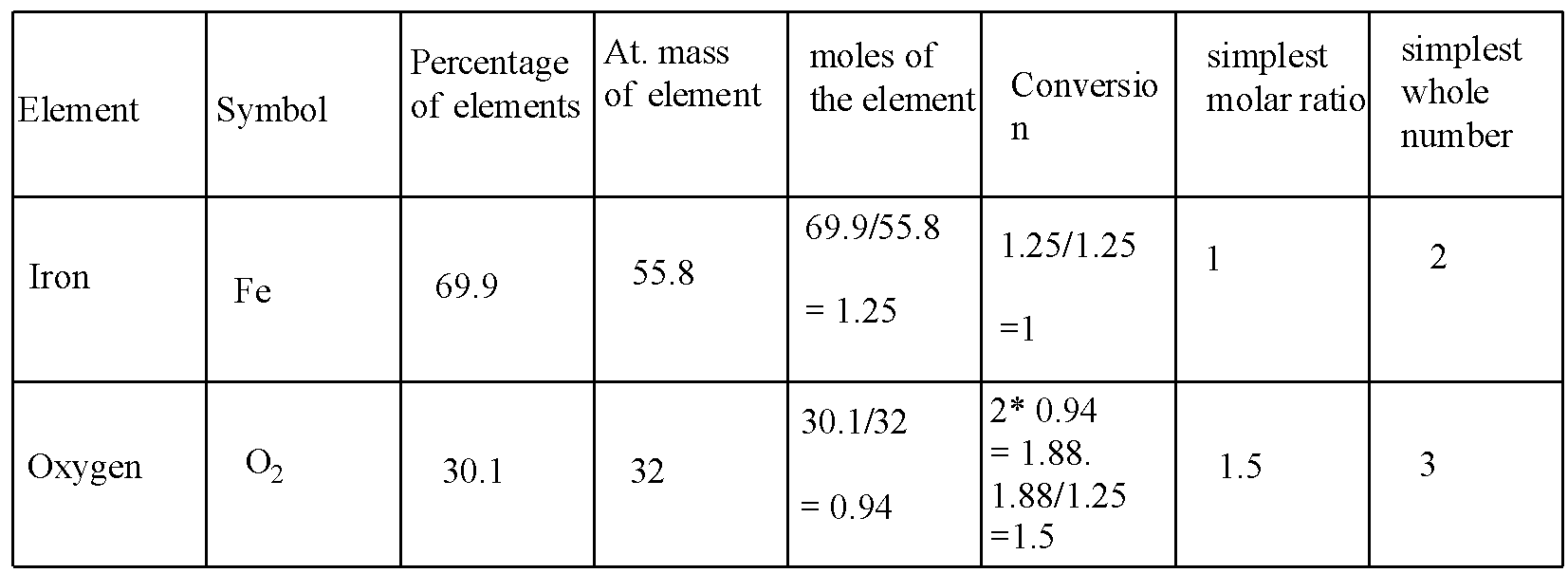
In the conversion, the 0.94 is multiplied with 2 because the oxygen is diatomic.
Hence, the formula is \[F{{e}_{2}}{{O}_{3}}\] .
Note:
The simplest molecular ration should always be converted into the whole number. If not converted this would give the exact or true formula which gives the molecular formula. Don't get confused between molecular and empirical formulas.
Complete step by step answer:
The empirical formula is the chemical formula that tells the simplest whole-number ratio of the atoms of all elements present in one molecule.
It is deduced by- (a) percentage composition of elements (b)- atomic masses.
There are some steps for calculating the empirical formula:
i)- To convert the mass percent into grams. The given mass percentage represents the masses of the element in grams.
ii)- To calculate the number of moles. Divide the percentage of each element by its atomic mass.
\[\text{moles of the element= }\frac{\text{mass of the element}}{\text{At}\text{. mass of the element}}\]
iii)-To calculate the simplest molar ration. Divide the moles obtained in step (i) by the smallest quotient or the least value from amongst the values obtained for each element. This gives the simplest molar ratio.
iv)- To calculate the simplest whole number ratio. The simplest molar ratios, as calculated in step 2, are generally whole numbers. If not, then (a) raise the values to the nearest whole number, or (b) multiply the simplest atomic ratio by a suitable integer.
v)- To write the empirical formula. Insert the numerical value of the simplest whole number.
According to the following steps, for iron and oxygen is solved in the table:

In the conversion, the 0.94 is multiplied with 2 because the oxygen is diatomic.
Hence, the formula is \[F{{e}_{2}}{{O}_{3}}\] .
Note:
The simplest molecular ration should always be converted into the whole number. If not converted this would give the exact or true formula which gives the molecular formula. Don't get confused between molecular and empirical formulas.
Watch videos on
Determine the empirical formula of an oxide of iron which has 69.9% iron and 30.1% dioxygen by mass.

Some Basic Concepts of Chemistry | NCERT EXERCISE 1.3 | Class 11 Chemistry Chapter 1 | Nandini Ma'am
Subscribe
 Share
Share likes
40.5K Views
2 years ago
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE



 Watch Video
Watch Video
