
Describe the thistle funnel experiment to demonstrate osmosis with a labeled diagram.
Answer
571.5k+ views
Hint: In the thistle funnel experiment the increased level of water in the funnel is noted because if it increases or decreases that will prove the process occurred or not.
Complete answer:
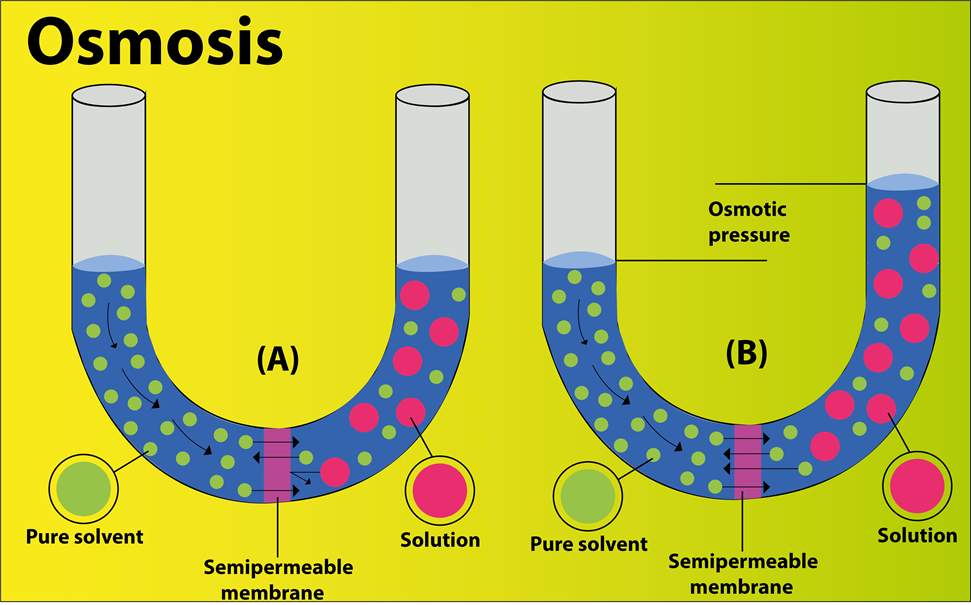
Osmosis is a process in which molecules of a solvent tend to pass through a semipermeable membrane from the region of higher concentration towards the region of lower concentration.
Following are the requirements of materials to demonstrate the process of osmosis by thistle funnel experiment:
Beaker
Thistle funnel
A sheet of cellophane or goat bladder
Thread
Sugar solution
Water
Methods of the experiment:
- first of all, take the glass tube and tie up a sheet of cellophane with the help of thread at the lower opening of the glass tube.
- Now prepare a highly concentrated sugar solution with the help of water.
- Then fill the interior of the glass tube with the concentrated sugar solution.
- Now place the entire setup of apparatus in a beaker containing distilled water.
- We will note down the reading in the thistle funnel i.e., the level of water should be noted.
- After some time we will observe that the label of water is increasing in the funnel. This means that the movement of water has taken place through the cellophane sheet inside the funnel.
- Concentration of water is ${ 100\% }$ in the beaker as compared to the sugar solution. Therefore, the water moves from the region of high concentration to the region of low concentration through a semipermeable membrane i.e., the cellophane sheet. Thus the experiment shows the phenomenon of osmosis.
Note:
- Thistle funnels are wont to add small volumes of liquids to a particular position. Thistle funnels are found with or without taps.
- The force, with which the answer level within the tube increases, arises from the pressure exerted by the diffusion of water molecules into the tube. This pressure is called osmotic pressure.
- Stability of the water level within the funnel indicates that water concentration in both the beakers also because the funnel is that the same and thus osmosis stops.
Complete answer:
Osmosis is a process in which molecules of a solvent tend to pass through a semipermeable membrane from the region of higher concentration towards the region of lower concentration.
Following are the requirements of materials to demonstrate the process of osmosis by thistle funnel experiment:
Beaker
Thistle funnel
A sheet of cellophane or goat bladder
Thread
Sugar solution
Water

Methods of the experiment:
- first of all, take the glass tube and tie up a sheet of cellophane with the help of thread at the lower opening of the glass tube.
- Now prepare a highly concentrated sugar solution with the help of water.
- Then fill the interior of the glass tube with the concentrated sugar solution.
- Now place the entire setup of apparatus in a beaker containing distilled water.
- We will note down the reading in the thistle funnel i.e., the level of water should be noted.
- After some time we will observe that the label of water is increasing in the funnel. This means that the movement of water has taken place through the cellophane sheet inside the funnel.
- Concentration of water is ${ 100\% }$ in the beaker as compared to the sugar solution. Therefore, the water moves from the region of high concentration to the region of low concentration through a semipermeable membrane i.e., the cellophane sheet. Thus the experiment shows the phenomenon of osmosis.
Note:
- Thistle funnels are wont to add small volumes of liquids to a particular position. Thistle funnels are found with or without taps.
- The force, with which the answer level within the tube increases, arises from the pressure exerted by the diffusion of water molecules into the tube. This pressure is called osmotic pressure.
- Stability of the water level within the funnel indicates that water concentration in both the beakers also because the funnel is that the same and thus osmosis stops.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Explain zero factorial class 11 maths CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

State and prove Bernoullis theorem class 11 physics CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE




