
Describe in words the atomic composition denoted by chemical formula ${S_8}$
Answer
573.6k+ views
Hint: To determine the atomic composition of this molecule, first we have to identify the element, by observing the element’s symbol. The atomic composition of this element is what describes to us the configuration, positioning and structure of the atoms present in this molecule. Thus, to describe the atomic composition, we can write about the shape of the molecule, the various forms it exists in and other physical properties like melting point and strain on the bonds present.
Complete step by step answer:
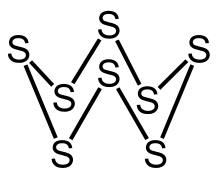
The given compound is the most commonly found structure of the element sulphur ($S$), having eight sulphur atoms which form a ring-like structure. Sulphur is a chemical element that is present in all living tissues. ${S_8}$ is the most commonly used industrial and pharmaceutical form of sulphur. After calcium and phosphorus, it is also the third most abundant mineral in the human body.
Now let us have a look at the shape and structure of this molecule.
Sulphur when present as octa-sulphur adopts a puckered ring or crown structure. This is due to the positioning of the eight corner sulphur atoms, which makes the cyclic structure look like a crown shaped structure. All the bonds between the eight atoms are covalent bonds.
We can describe the physical characteristics of this molecule as:
A molecule of sulphur is composed of eight sulphur atoms and is therefore written as ${S_8}$. This form of sulphur is commonly found as a yellow crystallized powder, as the molecule easily forms crystals, which are somewhat semi-transparent.
It is found that this molecule exists in different forms. We can describe them as follows:
The octa-sulphur molecule crystallizes in three distinct forms, namely: rhombohedral, and two monoclinic forms, of which only two are stable at standard conditions. The most common standard used when referring to ${S_8}$ is the rhombohedral form. The remaining form is only stable between $96^\circ C$ and $115\;^\circ C$, and that too at $100{{ }}kPa$. As a result, this molecule has three different melting and boiling points, corresponding to the three forms.
The common names given to this compound are octadecane and cyclo-octasulphur.
And finally, coming to the bond structure in this molecule, we see that:
In ${S_8}$ the bond angle between two bonds is small and they can withstand electronic effects because of the larger size of the $S$ atom, which makes repulsion between them weaker.
Note: Octasulfur is the most widely occurring allotrope of sulphur. Allotropes are different structural forms of the same element. Note that when rhombohedral sulphur is heated above $95.6^\circ C$, it changes its form into monoclinic sulphur; when cooled below $95.6^\circ C$, it reverts back to rhombic sulphur. Hence, $95.6^\circ C$ is known as the transition temperature of sulphur.
Complete step by step answer:
The given compound is the most commonly found structure of the element sulphur ($S$), having eight sulphur atoms which form a ring-like structure. Sulphur is a chemical element that is present in all living tissues. ${S_8}$ is the most commonly used industrial and pharmaceutical form of sulphur. After calcium and phosphorus, it is also the third most abundant mineral in the human body.
Now let us have a look at the shape and structure of this molecule.
Sulphur when present as octa-sulphur adopts a puckered ring or crown structure. This is due to the positioning of the eight corner sulphur atoms, which makes the cyclic structure look like a crown shaped structure. All the bonds between the eight atoms are covalent bonds.

We can describe the physical characteristics of this molecule as:
A molecule of sulphur is composed of eight sulphur atoms and is therefore written as ${S_8}$. This form of sulphur is commonly found as a yellow crystallized powder, as the molecule easily forms crystals, which are somewhat semi-transparent.
It is found that this molecule exists in different forms. We can describe them as follows:
The octa-sulphur molecule crystallizes in three distinct forms, namely: rhombohedral, and two monoclinic forms, of which only two are stable at standard conditions. The most common standard used when referring to ${S_8}$ is the rhombohedral form. The remaining form is only stable between $96^\circ C$ and $115\;^\circ C$, and that too at $100{{ }}kPa$. As a result, this molecule has three different melting and boiling points, corresponding to the three forms.
The common names given to this compound are octadecane and cyclo-octasulphur.
And finally, coming to the bond structure in this molecule, we see that:
In ${S_8}$ the bond angle between two bonds is small and they can withstand electronic effects because of the larger size of the $S$ atom, which makes repulsion between them weaker.
Note: Octasulfur is the most widely occurring allotrope of sulphur. Allotropes are different structural forms of the same element. Note that when rhombohedral sulphur is heated above $95.6^\circ C$, it changes its form into monoclinic sulphur; when cooled below $95.6^\circ C$, it reverts back to rhombic sulphur. Hence, $95.6^\circ C$ is known as the transition temperature of sulphur.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




