
Deprotonation will occur in which of the following positions?
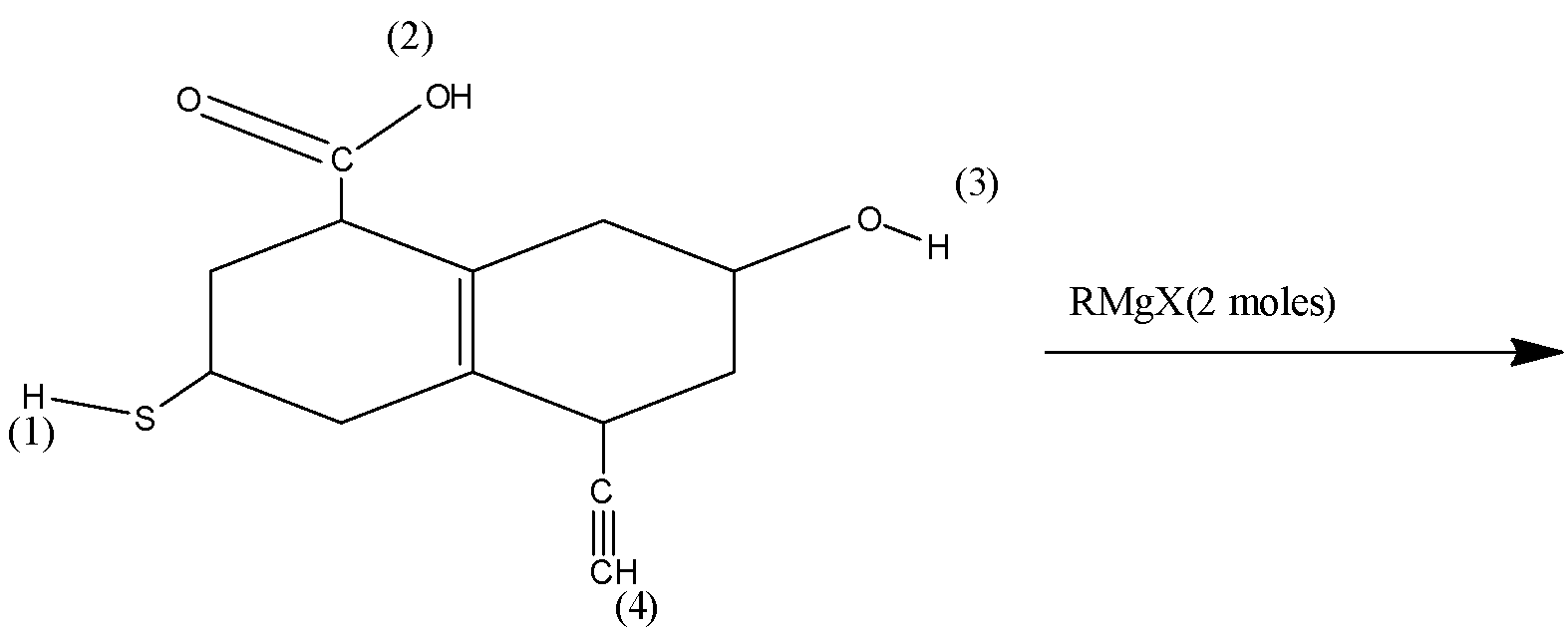
A) ${\text{1}}$ and ${\text{2}}$
B) ${\text{1}}$ and ${\text{3}}$
C) ${\text{1 and 4}}$
D) Any two positions

Answer
573.6k+ views
Hint:Deprotonation is a reaction in which a hydrogen atom is removed from a particular group in structure. The hydrogen when removed forms an anion and the anion formed which is stabilized will be deprotonated first.
Complete answer:
1) First of all we will learn about the concept of deprotonation and which group will deprotonate faster present in the structure. The deprotonation means the removal of the proton that is hydrogen from a structure.
2) When the proton that is hydrogen is removed from the group present in a structure it will form an anion on the atom which was attached to the hydrogen. The stability of the anion which is formed after the deprotonation will give a faster reaction of deprotonation.
3) Now let the groups given in the structure which can donate the proton that is hydrogen and stability of the anion formed. The number (1) group is ${\text{S - H}}$ which will be forming an anion of ${{\text{S}}^ - }$ which is a stable anion due to the more electronegativity of the sulfur atom.
4) The number (2) group is an acid functional group ${\text{ - COOH}}$ that will be forming an anion of ${\text{ - CO}}{{\text{O}}^ - }$ and this one is the most stable anionic form as the anion is stabilized by the resonance of the carbonyl group.
5) The number (3) group is an alcoholic group that will form an anion of ${{\text{O}}^ - }$ and this form is relatively less stable as oxygen has less electronegativity than the sulfur atom. The number (4) group is the acetylene group which will form as the anion of ${\text{ - C}} \equiv {{\text{C}}^ - }$ which is a highly unstable form of anion due to triple bond.
6) If we write the order of the deprotonation it will be,
$COOH > SH > OH > HC \equiv CH$
Therefore, deprotonation will occur first at ${\text{1}}$ and ${\text{2}}$ positions which show option A as the correct choice.
Note:
The anion is an electron cloud which is stabilized by an electronegative atom or resonance present in the structure. The more stable structure of the anion the more it will try to get that stable state and faster it will try to donate a proton. The concept of deprotonation is similar to the concept of acidity.
Complete answer:
1) First of all we will learn about the concept of deprotonation and which group will deprotonate faster present in the structure. The deprotonation means the removal of the proton that is hydrogen from a structure.
2) When the proton that is hydrogen is removed from the group present in a structure it will form an anion on the atom which was attached to the hydrogen. The stability of the anion which is formed after the deprotonation will give a faster reaction of deprotonation.
3) Now let the groups given in the structure which can donate the proton that is hydrogen and stability of the anion formed. The number (1) group is ${\text{S - H}}$ which will be forming an anion of ${{\text{S}}^ - }$ which is a stable anion due to the more electronegativity of the sulfur atom.
4) The number (2) group is an acid functional group ${\text{ - COOH}}$ that will be forming an anion of ${\text{ - CO}}{{\text{O}}^ - }$ and this one is the most stable anionic form as the anion is stabilized by the resonance of the carbonyl group.
5) The number (3) group is an alcoholic group that will form an anion of ${{\text{O}}^ - }$ and this form is relatively less stable as oxygen has less electronegativity than the sulfur atom. The number (4) group is the acetylene group which will form as the anion of ${\text{ - C}} \equiv {{\text{C}}^ - }$ which is a highly unstable form of anion due to triple bond.
6) If we write the order of the deprotonation it will be,
$COOH > SH > OH > HC \equiv CH$
Therefore, deprotonation will occur first at ${\text{1}}$ and ${\text{2}}$ positions which show option A as the correct choice.
Note:
The anion is an electron cloud which is stabilized by an electronegative atom or resonance present in the structure. The more stable structure of the anion the more it will try to get that stable state and faster it will try to donate a proton. The concept of deprotonation is similar to the concept of acidity.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




