
Define an atomic orbital.
Answer
571.8k+ views
Hint: An atom is the smallest unit of matter which forms a chemical element. Every solid, liquid, gas consists of atoms. Atoms consist of protons, neutrons and electrons.
Complete answer:
Atomic orbitals are the 3-dimensional spaces around the nucleus where the probability of finding an electron is maximum. The molecules orbitals are formed by combining the atomic orbitals. In quantum chemistry we have encounter orbitals which are s, p, d and f subshells. Orbitals are of different shapes and sizes and they can be determined by the square of the wave function.
Shapes of orbitals s, p, d and f are given below:
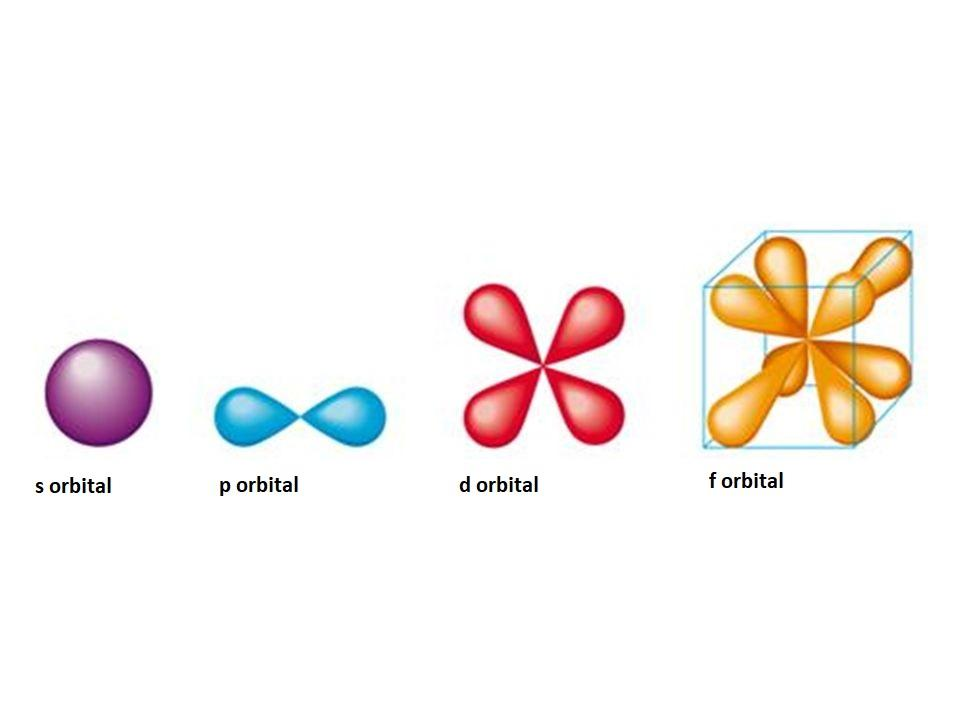
Orbitals are of different shapes the s orbital has a spherical shape, the p orbital has a dumbbell shape the d orbital has a double dumbbell shape. The maximum number of electrons which can be present in an orbital is two. Almost all the orbitals are directional in nature except the s orbital, it is non directional in nature. The concept of orbitals is explained by the Heisenberg Uncertainty principle.
Note:
Do not get confused between orbit and orbitals. Orbit is a well-defined circular path around the nucleus in which the electrons revolve whereas atomic orbitals are the 3-dimensional spaced around the nucleus where the probability of finding an electron is maximum.
Orbits represent the motion of electrons in a plane and they are non-directional in nature. The orbits are either circular or elliptical in shape. The nodal plane is a plane where the probability of finding an electron is zero.
Complete answer:
Atomic orbitals are the 3-dimensional spaces around the nucleus where the probability of finding an electron is maximum. The molecules orbitals are formed by combining the atomic orbitals. In quantum chemistry we have encounter orbitals which are s, p, d and f subshells. Orbitals are of different shapes and sizes and they can be determined by the square of the wave function.
Shapes of orbitals s, p, d and f are given below:

Orbitals are of different shapes the s orbital has a spherical shape, the p orbital has a dumbbell shape the d orbital has a double dumbbell shape. The maximum number of electrons which can be present in an orbital is two. Almost all the orbitals are directional in nature except the s orbital, it is non directional in nature. The concept of orbitals is explained by the Heisenberg Uncertainty principle.
Note:
Do not get confused between orbit and orbitals. Orbit is a well-defined circular path around the nucleus in which the electrons revolve whereas atomic orbitals are the 3-dimensional spaced around the nucleus where the probability of finding an electron is maximum.
Orbits represent the motion of electrons in a plane and they are non-directional in nature. The orbits are either circular or elliptical in shape. The nodal plane is a plane where the probability of finding an electron is zero.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




