
DBN is a bicyclic compound which is used as a base. What is the major product in the following reaction?
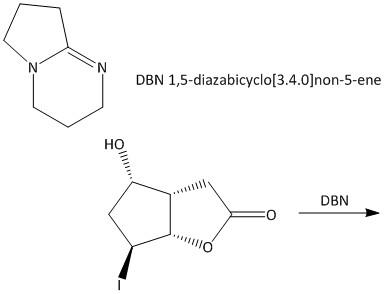
A.
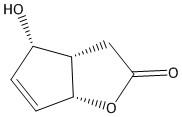
B.
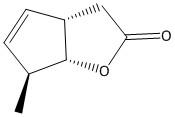
C.
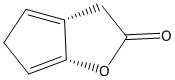
D. Both A and B




Answer
573.3k+ views
Hint: \[DBN\]is a fused bicyclic compound formed by the fusion of a five and a six membered ring. This compound acts as an organic base in organic synthesis.
Complete step by step answer:
\[DBN\] is an organic compound with chemical formula \ [{C_7}{H_{12}}{N_2}\]. It is an amidine base and is often used for abstracting protons from compounds. The anion produced takes part in several reactions like elimination, substitution, addition and rearrangement reactions.
The given reaction is an example of elimination reaction. The reaction begins by abstracting the proton by the base. The anion produced takes part in an elimination reaction by removal of the iodo group. The iodo group is a very good leaving group.
Iodine is an element in the periodic table with atomic number\[53\]. Its electronic configuration is $I:[Kr]4{d^{10}}5{s^2}5{p^5}$
The iodine has vacant \[p\]-orbital and can place an electron in the orbital. Iodine also has inner \[4f\] orbitals in which it can accommodate an extra pair of electrons. The mechanism for the elimination reaction is written as:
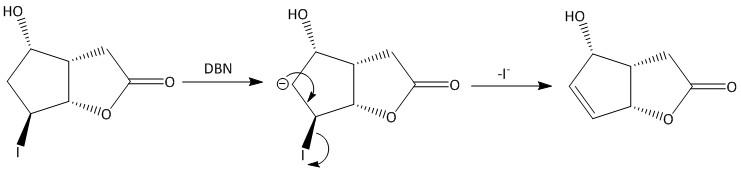
The endocyclic double bond is formed by the elimination of iodo groups. The reaction produces two \[s{p^2}\] hybridized carbon atoms from \[s{p^3}\] hybridized carbon atom. The reaction follows \[E1cb\](\[cb\] = conjugate base) mechanism in which the first step is the rate determining step, i.e. anion formation. The first step is reversible in nature. The second step is a slow step.
So, the correct answer is Option A.
Note: The reaction is also done using other bases like \[DBU\] or triethylamine or\[DIPEA\]. All these are organic bases and help in abstraction of protons and thereby resulting in an elimination reaction.
Complete step by step answer:
\[DBN\] is an organic compound with chemical formula \ [{C_7}{H_{12}}{N_2}\]. It is an amidine base and is often used for abstracting protons from compounds. The anion produced takes part in several reactions like elimination, substitution, addition and rearrangement reactions.
The given reaction is an example of elimination reaction. The reaction begins by abstracting the proton by the base. The anion produced takes part in an elimination reaction by removal of the iodo group. The iodo group is a very good leaving group.
Iodine is an element in the periodic table with atomic number\[53\]. Its electronic configuration is $I:[Kr]4{d^{10}}5{s^2}5{p^5}$
The iodine has vacant \[p\]-orbital and can place an electron in the orbital. Iodine also has inner \[4f\] orbitals in which it can accommodate an extra pair of electrons. The mechanism for the elimination reaction is written as:

The endocyclic double bond is formed by the elimination of iodo groups. The reaction produces two \[s{p^2}\] hybridized carbon atoms from \[s{p^3}\] hybridized carbon atom. The reaction follows \[E1cb\](\[cb\] = conjugate base) mechanism in which the first step is the rate determining step, i.e. anion formation. The first step is reversible in nature. The second step is a slow step.
So, the correct answer is Option A.
Note: The reaction is also done using other bases like \[DBU\] or triethylamine or\[DIPEA\]. All these are organic bases and help in abstraction of protons and thereby resulting in an elimination reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




