
How many cyclic isomers of ${{C}_{5}}{{H}_{10}}$ are possible? (Consider structural as well as geometrical isomers)
A.6
B.7
C.8
D.9
Answer
577.8k+ views
Hint: Among the cyclic isomers of the compound, in order to find the stereoisomers, the carbon with the chiral centre is needed to be identified. Then with respect to the chiral carbon, we can find the other isomers.
Complete step by step solution:
In order to answer our question, let us know what isomerism is. Isomerism is the phenomenon by which two or more compounds that have the same molecular formula but have different chemical and physical properties. In structural isomerism, compounds have the same molecular formula but different structural formulae. Whereas, the compounds having same molecular as well as same structural formulae but differing in the relative arrangement of atoms or groups in space are called stereoisomers and this phenomenon is called stereomerism. Let us find the degree of unsaturation for the compound. Degree of unsaturation is given by:
$D.U=C-\dfrac{H}{2}+1$
So, $D.{{U}_{{{C}_{5}}{{H}_{10}}}}=5-\dfrac{10}{2}+1=1$
As the DBE is 1, it means that either there is one ring or one unsaturation I,e double bond. However, we will find the cyclic or ring structures only. Now, we can form either a 3,4 or maximum 5 membered ring. Let us make a five membered ring first.
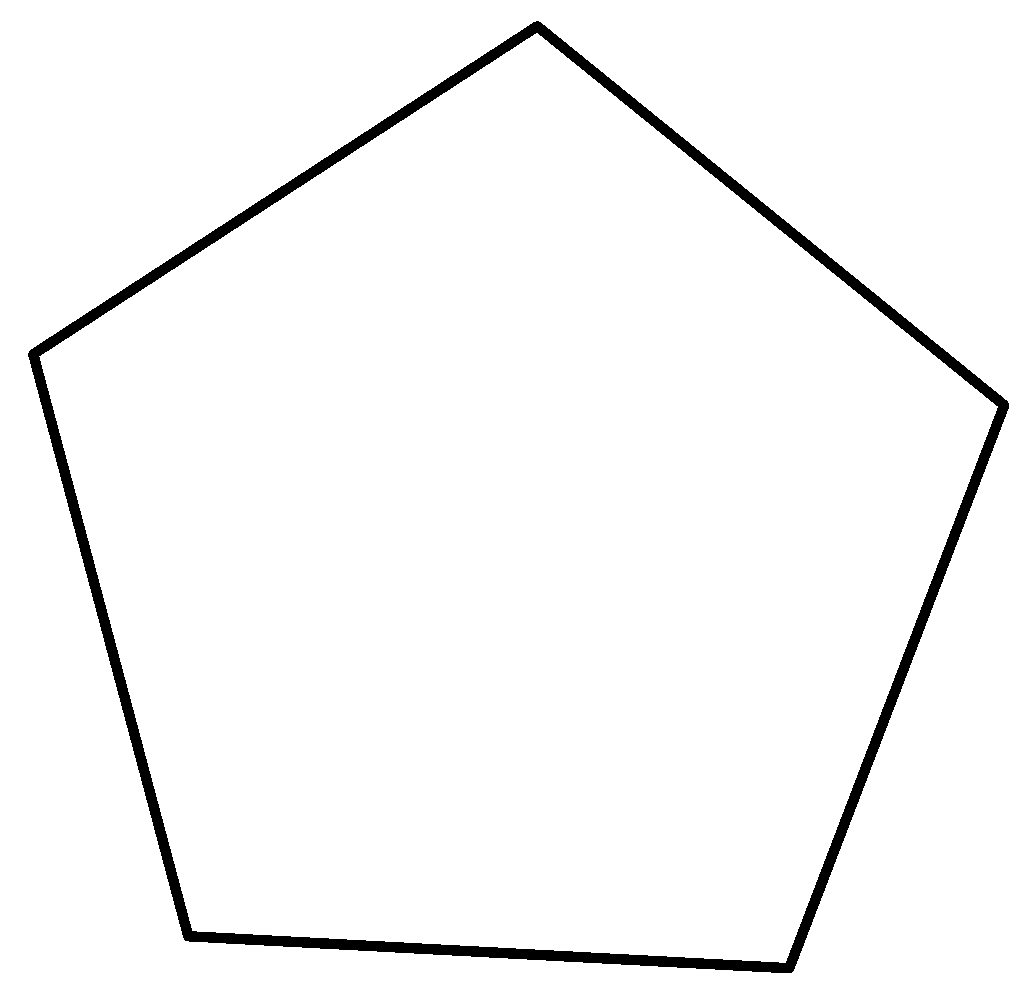
This is the only option we have for a five-membered ring. Let us draw a four membered ring now.
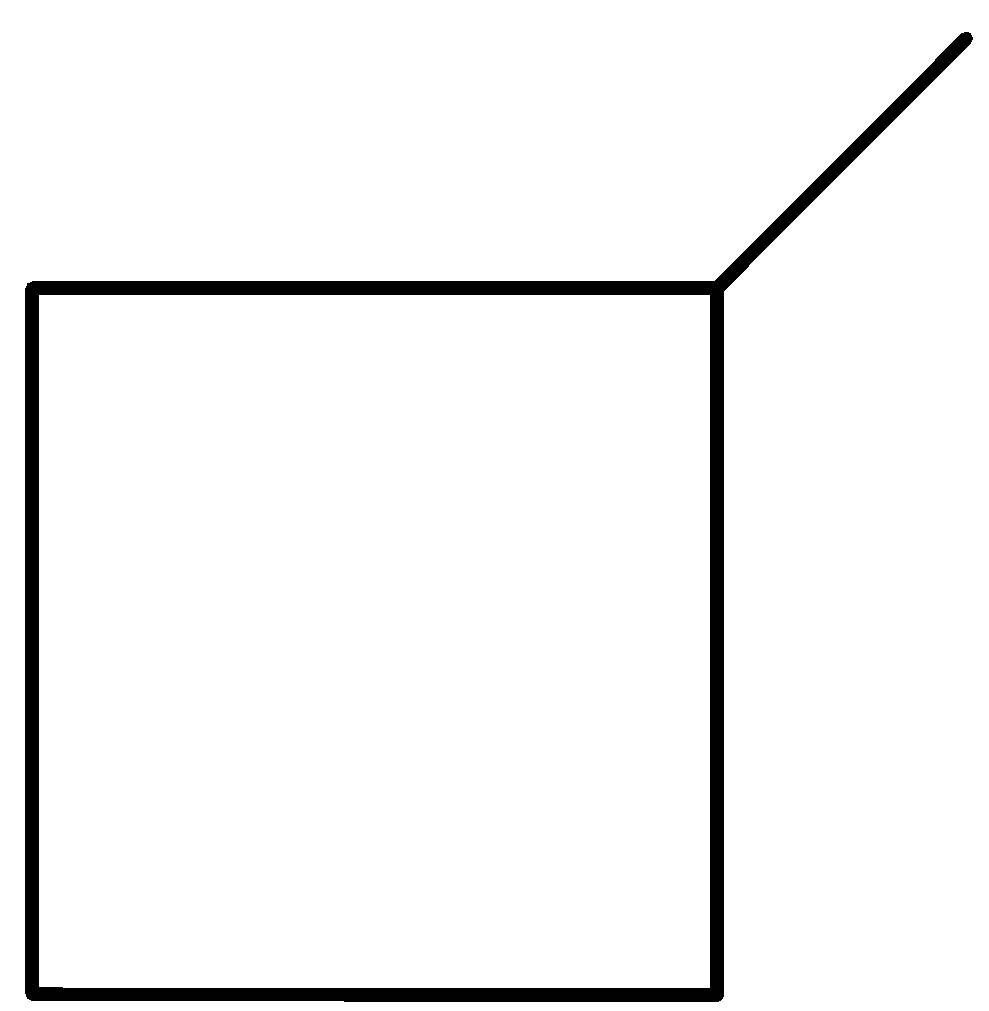
Even here, we are left with only one option. Let us see the case of 3 membered rings:
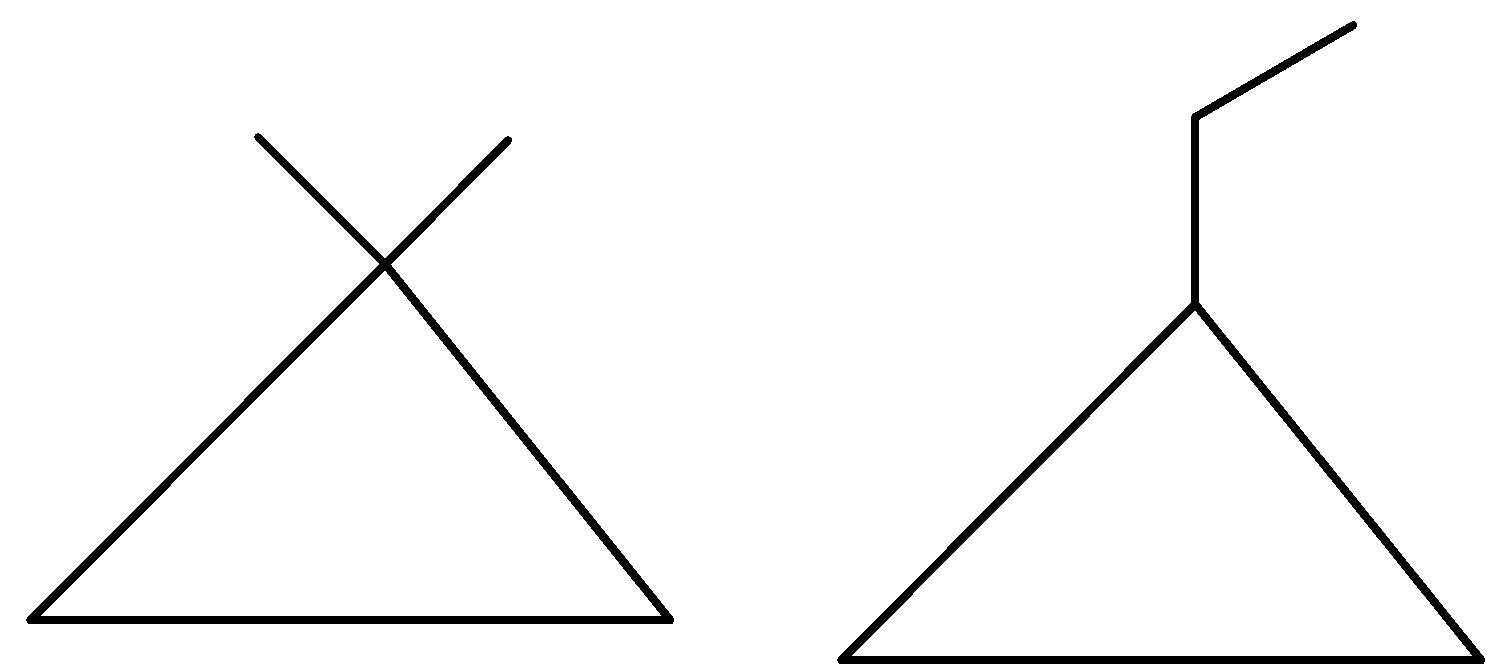
We can either attach the two methyl groups on one carbon side by side or join them as an ethyl group. However, there is one more possibility where we attach two methyl groups on separate carbons.
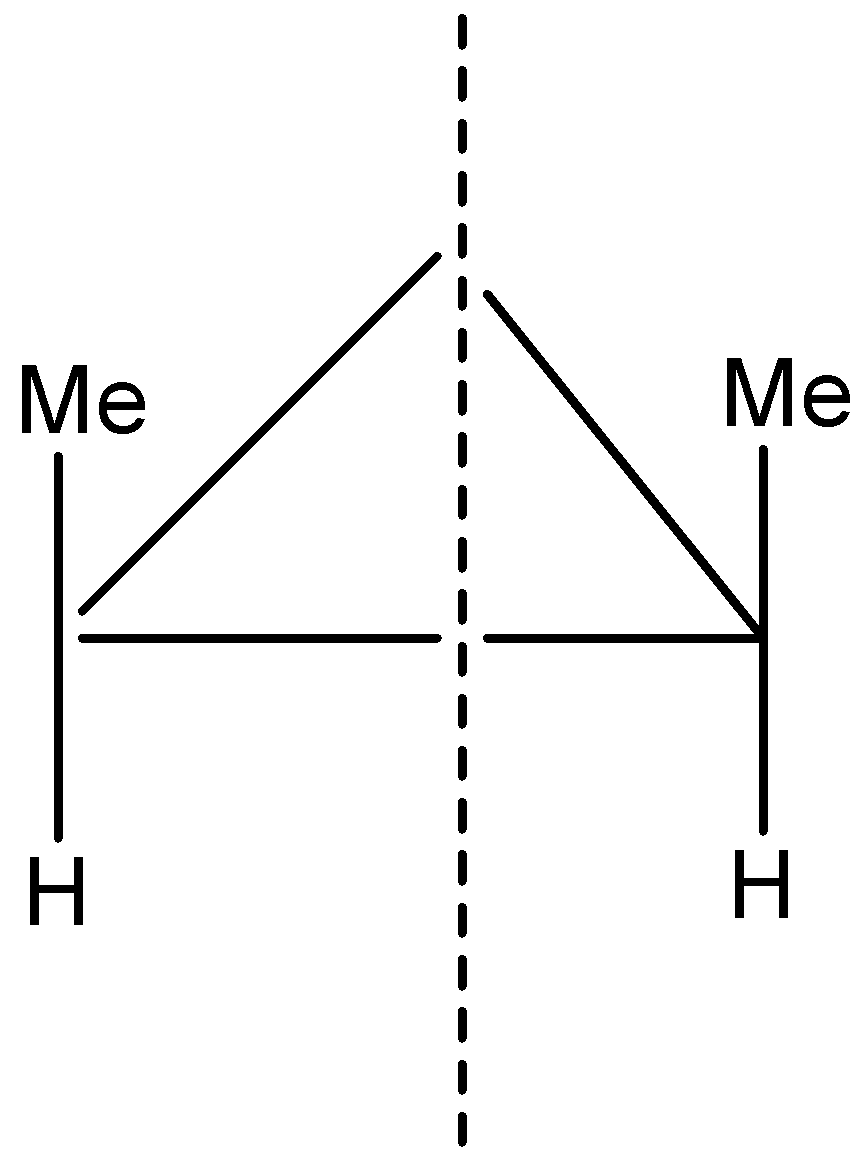
The compound looks like this, but it is a meso compound and has a plane of symmetry. So one more structure can be:
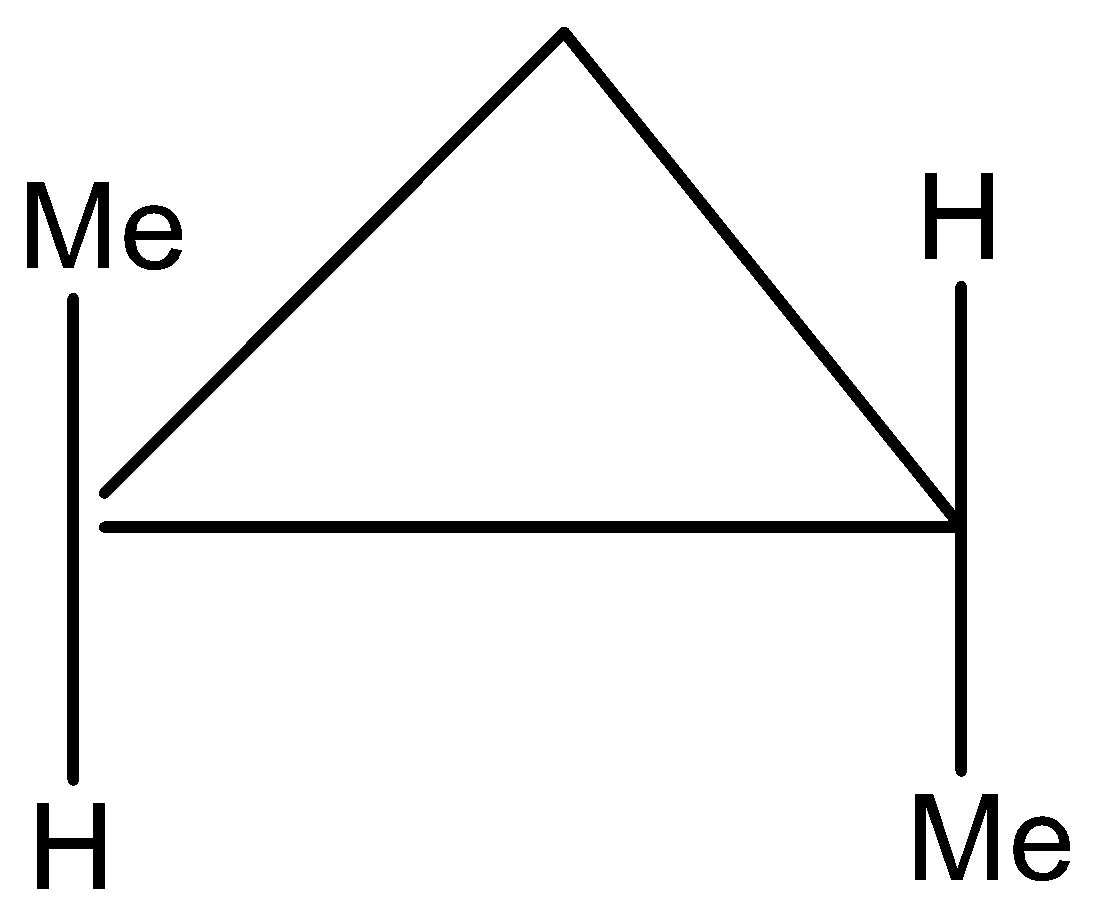
This compound above, is an enantiomer and also has a mirror image of itself, which looks like:
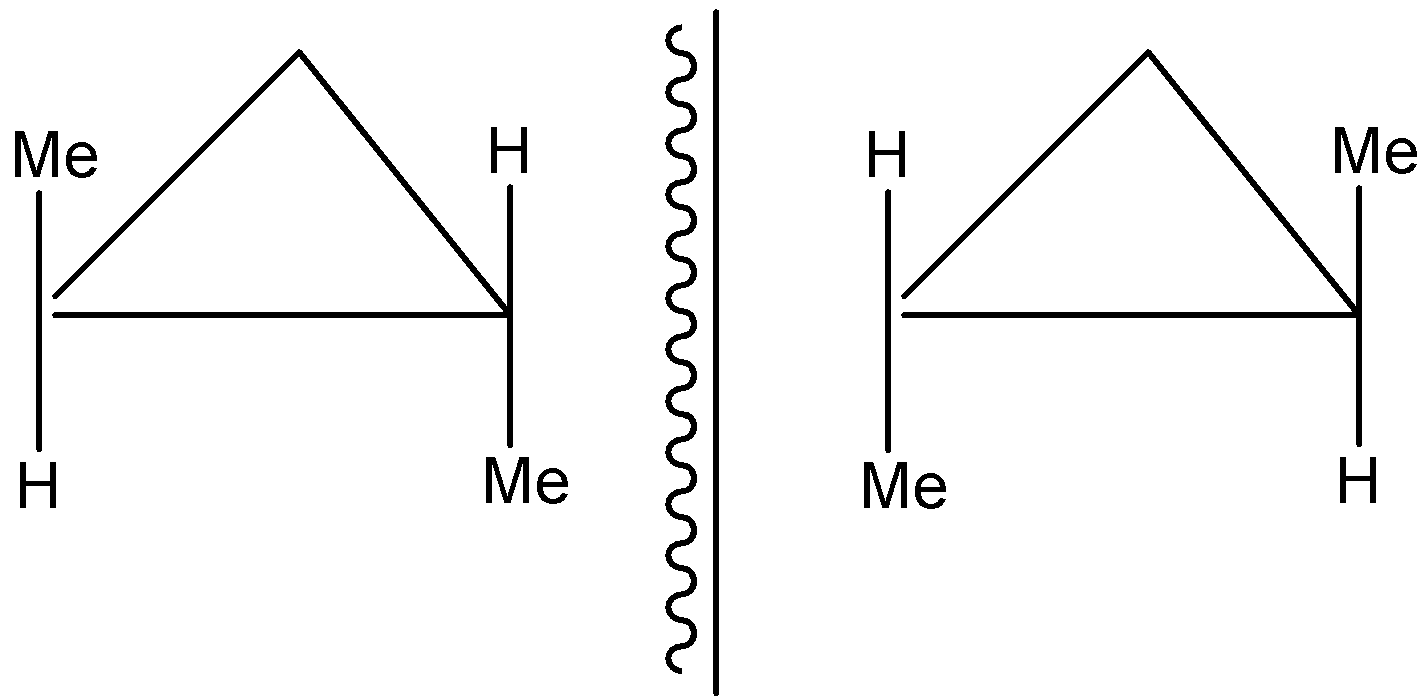
So, in total, we have 7 cyclic isomers, including stereoisomers and geometrical isomers.
So, the correct answer is option B.
NOTE: In stereomerism, when the same groups are present on the same side, in this case Me and H, then it is called a cis isomer and when groups are present on opposite sides, then it is called a trans isomer. Generally, trans isomers are more stable than cis isomers.
Complete step by step solution:
In order to answer our question, let us know what isomerism is. Isomerism is the phenomenon by which two or more compounds that have the same molecular formula but have different chemical and physical properties. In structural isomerism, compounds have the same molecular formula but different structural formulae. Whereas, the compounds having same molecular as well as same structural formulae but differing in the relative arrangement of atoms or groups in space are called stereoisomers and this phenomenon is called stereomerism. Let us find the degree of unsaturation for the compound. Degree of unsaturation is given by:
$D.U=C-\dfrac{H}{2}+1$
So, $D.{{U}_{{{C}_{5}}{{H}_{10}}}}=5-\dfrac{10}{2}+1=1$
As the DBE is 1, it means that either there is one ring or one unsaturation I,e double bond. However, we will find the cyclic or ring structures only. Now, we can form either a 3,4 or maximum 5 membered ring. Let us make a five membered ring first.

This is the only option we have for a five-membered ring. Let us draw a four membered ring now.

Even here, we are left with only one option. Let us see the case of 3 membered rings:
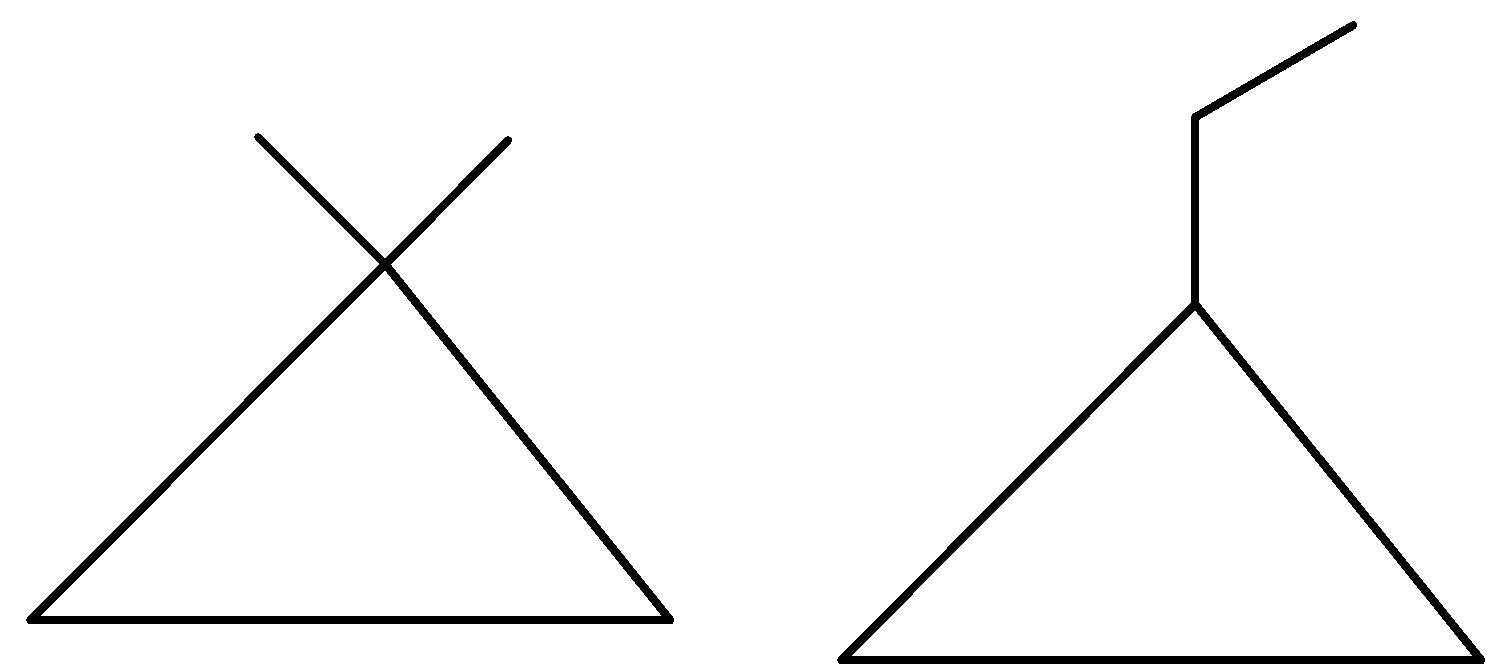
We can either attach the two methyl groups on one carbon side by side or join them as an ethyl group. However, there is one more possibility where we attach two methyl groups on separate carbons.

The compound looks like this, but it is a meso compound and has a plane of symmetry. So one more structure can be:

This compound above, is an enantiomer and also has a mirror image of itself, which looks like:

So, in total, we have 7 cyclic isomers, including stereoisomers and geometrical isomers.
So, the correct answer is option B.
NOTE: In stereomerism, when the same groups are present on the same side, in this case Me and H, then it is called a cis isomer and when groups are present on opposite sides, then it is called a trans isomer. Generally, trans isomers are more stable than cis isomers.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




