
What is the covalency of nitrogen in \[{{N}_{2}}{{O}_{5}}\]?
Answer
530k+ views
Hint: The number of electrons present in the outermost shell that is shared by an atom in a compound is called covalency. In nitrogen pentoxide, there are two nitrogen and five oxygen atoms.
Complete step by step solution:
The chemical activity of an atom depends on the number of electrons in its outermost shell. If the shells are packed fully to its capacity, then the atom is inert. If there is deficiency, then there will be a tendency in reaching that capacity. To reach this capacity, electrons are accepted, donated or shared between other atoms.
We know that the number of electrons in the valence shell is called the valency of the atom. In an ionic compound or where there is donation and acceptance of electrons, the valency is called electrovalency.
When sharing of electrons occurs between two atoms, a covalent bond is formed between them and the valency of each atom is termed as covalency. Carbon has four electrons in its valence shell and requires four more electrons. Hydrogen has an electron in its valence shell. So, carbon can share its four electrons with four hydrogen atoms. SO the covalency of carbon is four.
Similarly, in nitrogen pentoxide, there are two nitrogen and five oxygen atoms. Each nitrogen is bonded to 3 oxygen. In which, one of the oxygen is doubly bonded to the nitrogen. The two nitrogens are connected through oxygen. So, each nitrogen forms three covalent bonds with oxygen atoms and one coordination bond with one of the three oxygen atoms. Here nitrogen acquires a positive charge on it. Thus, nitrogen forms 4 bonds. Covalency is 4.
The structure of nitrogen pentoxide is
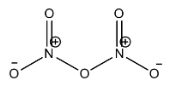
Therefore, the covalency of nitrogen in nitrogen pentoxide is 4.
Note: Covalency and electrovalency are different. Covalency is for covalent bonds and coordination bonds and electrovalency is for ionic bonds. Covalency is the number of electrons an atom can share while electrovalency is the number of electrons that an atom can gain or lose.
Complete step by step solution:
The chemical activity of an atom depends on the number of electrons in its outermost shell. If the shells are packed fully to its capacity, then the atom is inert. If there is deficiency, then there will be a tendency in reaching that capacity. To reach this capacity, electrons are accepted, donated or shared between other atoms.
We know that the number of electrons in the valence shell is called the valency of the atom. In an ionic compound or where there is donation and acceptance of electrons, the valency is called electrovalency.
When sharing of electrons occurs between two atoms, a covalent bond is formed between them and the valency of each atom is termed as covalency. Carbon has four electrons in its valence shell and requires four more electrons. Hydrogen has an electron in its valence shell. So, carbon can share its four electrons with four hydrogen atoms. SO the covalency of carbon is four.
Similarly, in nitrogen pentoxide, there are two nitrogen and five oxygen atoms. Each nitrogen is bonded to 3 oxygen. In which, one of the oxygen is doubly bonded to the nitrogen. The two nitrogens are connected through oxygen. So, each nitrogen forms three covalent bonds with oxygen atoms and one coordination bond with one of the three oxygen atoms. Here nitrogen acquires a positive charge on it. Thus, nitrogen forms 4 bonds. Covalency is 4.
The structure of nitrogen pentoxide is

Therefore, the covalency of nitrogen in nitrogen pentoxide is 4.
Note: Covalency and electrovalency are different. Covalency is for covalent bonds and coordination bonds and electrovalency is for ionic bonds. Covalency is the number of electrons an atom can share while electrovalency is the number of electrons that an atom can gain or lose.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




