
What is the correct option for Nylon-6,6?
a.) It is a natural polymer
b.) It’s monomeric unit is ethylene diamine
c.) It has amide linkage
d.) It has glycosidic linkage
Answer
583.2k+ views
Hint: Nylon is synthetic fiber. Nylon does not have glycosidic linkage. Nylon 6,6 is synthesized from two monomers, adipic acid and hexamethylenediamine. When carboxyl groups and amine groups lose water molecules, CO-NH linkage is formed.
Complete step by step answer:
Nylon name is derived as follows:
Ny indicates New York and lon indicates London. It was synthesized by man; it is first completely synthesized fiber as no natural raw material was used in its preparation.
6 in Nylon 6,6 indicates the number of carbon present in each monomer. Two monomers are used to synthesize Nylon-6,6. Monomers are Adipic acid and Hexamethylenediamine.
Preparation of Nylon 6,6 is as follows:
Carboxylic acid group COOH of adipic acid and Amino group of Hexamethylene diamine undergo condensation and loses water molecule and forms amide linkage then polymerization leads to formation of Nylon-6,6.
This condensation polymerization takes place in presence of high pressure and temperature.
-Nylon has high tensile strength. Nylon 6,6 has intermolecular forces like hydrogen bonding and it has crystalline nature.
-It has applications in textile industries, in making sheets, toothbrushes etc.
( C) it has amide linkage is the correct option for Nylon-6,6.

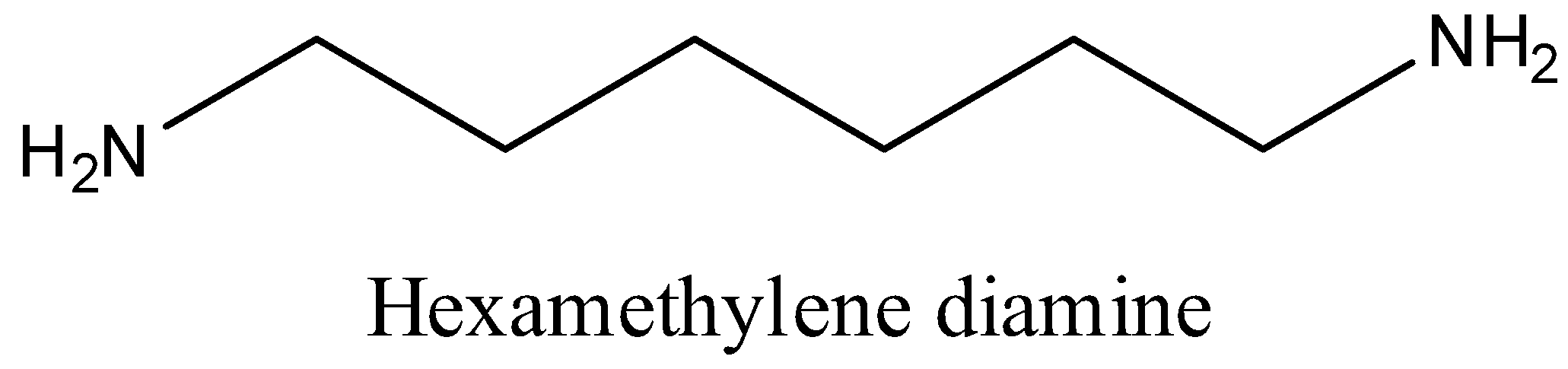
Note: In Nylon 6, Caprolactum is monomer. In Nylon 6,6 monomers used are Adipic acid and Hexamethylenediamine. Carboxylic acid group COOH of adipic acid and Amino group of Hexamethylene diamine undergo condensation and loses water molecule and forms amide linkage then polymerization leads to formation of Nylon-6,6.
Complete step by step answer:
Nylon name is derived as follows:
Ny indicates New York and lon indicates London. It was synthesized by man; it is first completely synthesized fiber as no natural raw material was used in its preparation.
6 in Nylon 6,6 indicates the number of carbon present in each monomer. Two monomers are used to synthesize Nylon-6,6. Monomers are Adipic acid and Hexamethylenediamine.
Preparation of Nylon 6,6 is as follows:
Carboxylic acid group COOH of adipic acid and Amino group of Hexamethylene diamine undergo condensation and loses water molecule and forms amide linkage then polymerization leads to formation of Nylon-6,6.
This condensation polymerization takes place in presence of high pressure and temperature.
-Nylon has high tensile strength. Nylon 6,6 has intermolecular forces like hydrogen bonding and it has crystalline nature.
-It has applications in textile industries, in making sheets, toothbrushes etc.
( C) it has amide linkage is the correct option for Nylon-6,6.


Note: In Nylon 6, Caprolactum is monomer. In Nylon 6,6 monomers used are Adipic acid and Hexamethylenediamine. Carboxylic acid group COOH of adipic acid and Amino group of Hexamethylene diamine undergo condensation and loses water molecule and forms amide linkage then polymerization leads to formation of Nylon-6,6.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




