
How do you convert the following:
Ethyl alcohol to ethoxy ethane.
Acetone to 2-methyl-2-propanol.
Answer
573.6k+ views
Hint: Ethers are prepared by the intermolecular dehydration of alcohols following the SN2 mechanism. We know that carbonyl carbons are generally converted to alcohols using Grignard reagent.
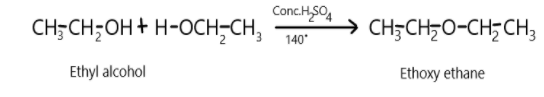
Complete Step by step answer: In the first conversion we need to know the dehydration of alcohols in the presence of protic acids at ${140^ \circ }C$will result in the formation of ether and it is a nucleophilic bimolecular reaction. so when ethyl alcohol will react with concentrated sulphuric acid at a temperature of ${140^ \circ }C$, it will result in the formation of ethoxy ethane which is our desired product and liberation of water molecules. This can be depicted as:
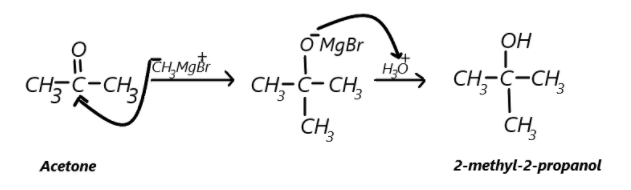
In the second compound we are to convert acetone which has a ketonic group thus a carbonyl carbon and we have to convert this carbonyl compound to alcohol, this can be done by using Grignard reagent which has a general formula $R - MgX$which will ionise as the alkyl part has a negative charge which will attack the carbonyl carbon. Here we can take $C{H_3} - MgBr$as Grignard reagent, so methyl will attack the carbonyl carbon and electrons from double bond will shift to oxygen and the product will have a $MgBr$attached to the oxygen, now if the hydrolysis take place in the presence of ${H_3}{O^ + }$ so hydrogen will be added in place of $MgBr$to the oxygen finally forming the desired product which is 2-methyl-2-propanol. This can be represented as:
Note: Ethoxy ethane can also be prepared using Williamson’s synthesis which is an easy and important method to prepare all types of ethers, in this method alkyl halide is allowed to react with sodium alkoxide and again it is a nucleophile substitution reaction which undergoes via SN2.
Complete Step by step answer: In the first conversion we need to know the dehydration of alcohols in the presence of protic acids at ${140^ \circ }C$will result in the formation of ether and it is a nucleophilic bimolecular reaction. so when ethyl alcohol will react with concentrated sulphuric acid at a temperature of ${140^ \circ }C$, it will result in the formation of ethoxy ethane which is our desired product and liberation of water molecules. This can be depicted as:

In the second compound we are to convert acetone which has a ketonic group thus a carbonyl carbon and we have to convert this carbonyl compound to alcohol, this can be done by using Grignard reagent which has a general formula $R - MgX$which will ionise as the alkyl part has a negative charge which will attack the carbonyl carbon. Here we can take $C{H_3} - MgBr$as Grignard reagent, so methyl will attack the carbonyl carbon and electrons from double bond will shift to oxygen and the product will have a $MgBr$attached to the oxygen, now if the hydrolysis take place in the presence of ${H_3}{O^ + }$ so hydrogen will be added in place of $MgBr$to the oxygen finally forming the desired product which is 2-methyl-2-propanol. This can be represented as:

Note: Ethoxy ethane can also be prepared using Williamson’s synthesis which is an easy and important method to prepare all types of ethers, in this method alkyl halide is allowed to react with sodium alkoxide and again it is a nucleophile substitution reaction which undergoes via SN2.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




