
: How will you convert the following?
A.Propan-2-ol to propanone
B.Phenol to 2, 4, 6-tribromophenol
Answer
591.6k+ views
Hint: Carbonyl compounds can be easily prepared from alcohols by their controlled oxidation.The polarization of bromine molecule in case of phenol takes place even in the absence of a Lewis acid and so phenols easily undergo electrophilic substitution reactions to give polysubstituted products.
Complete step by step answer:
A.Conversion of propan-2-ol to propanone:
On controlled oxidation, alcohols give aldehydes or ketones. Primary alcohols on controlled oxidation give aldehydes, whereas secondary alcohols on controlled oxidation give ketones.
The common oxidizing agents which are used for the purpose of converting the alcohols to aldehydes or ketones are acidified potassium dichromate which has the chemical formula ${{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}$ , aqueous or alkaline potassium permanganate which has the chemical formula ${\text{KMn}}{{\text{O}}_{\text{4}}}$ and chromic anhydride or chromium trioxide ${\text{Cr}}{{\text{O}}_{\text{3}}}$ and sulphuric acid, ${{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$ .
The general reaction of conversion of a primary alcohol to its corresponding aldehyde by oxidation using acidified potassium dichromate as oxidizing agent is shown below:
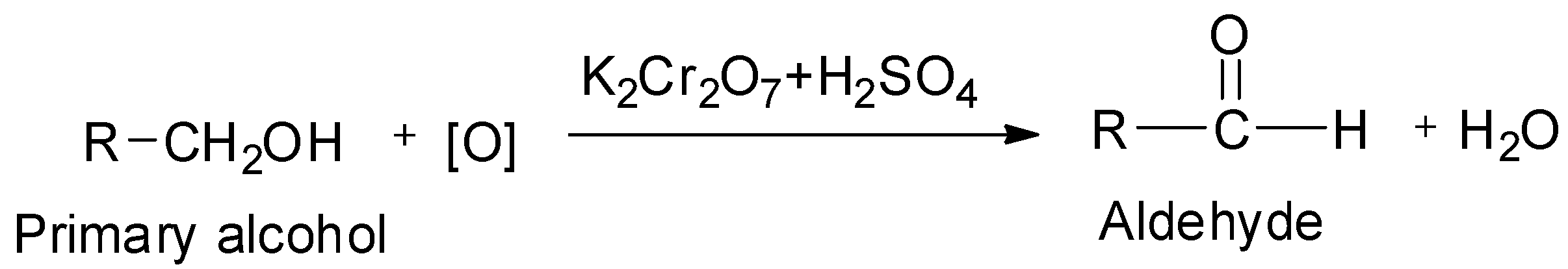
Now, propan-2-ol is a secondary alcohol as evident from its structure.

Therefore, propan-2-ol on oxidation with acidified potassium dichromate as an oxidizing agent will give its corresponding ketone. This ketone is propanone.
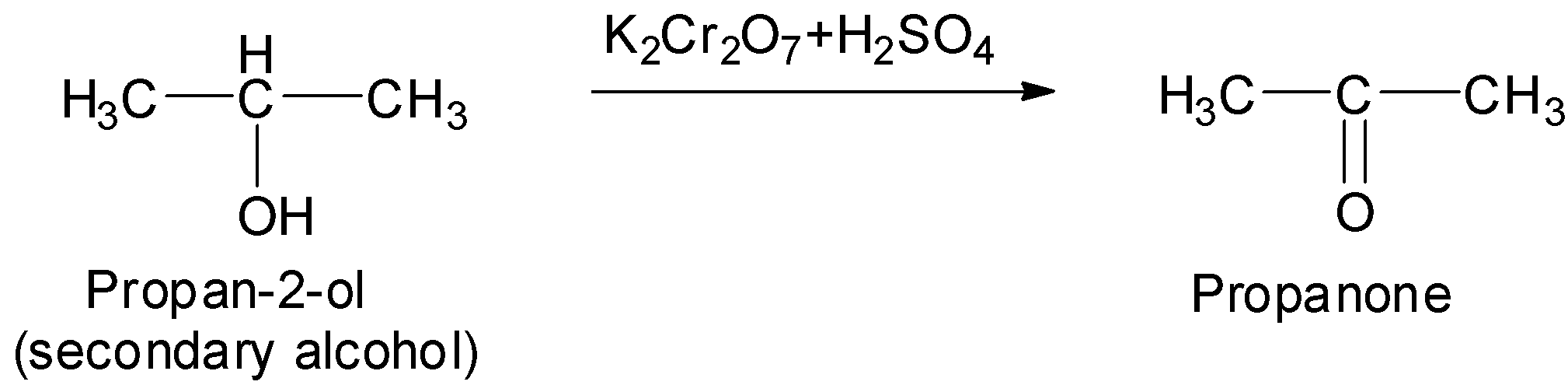
Thus, in this way we can convert propan-2-ol to propanone.
B.Conversion of phenol to 2, 4, 6-tribromophenol:
Due to the strong activating effect of the hydroxyl group attached to the benzene ring, halogenation of the benzene ring can take place even without a Lewis acid ultimately leading to polysubstitution. Moreover, the hydroxyl group is ortho, para directing and so it increases the electron density more at ortho and para positions.
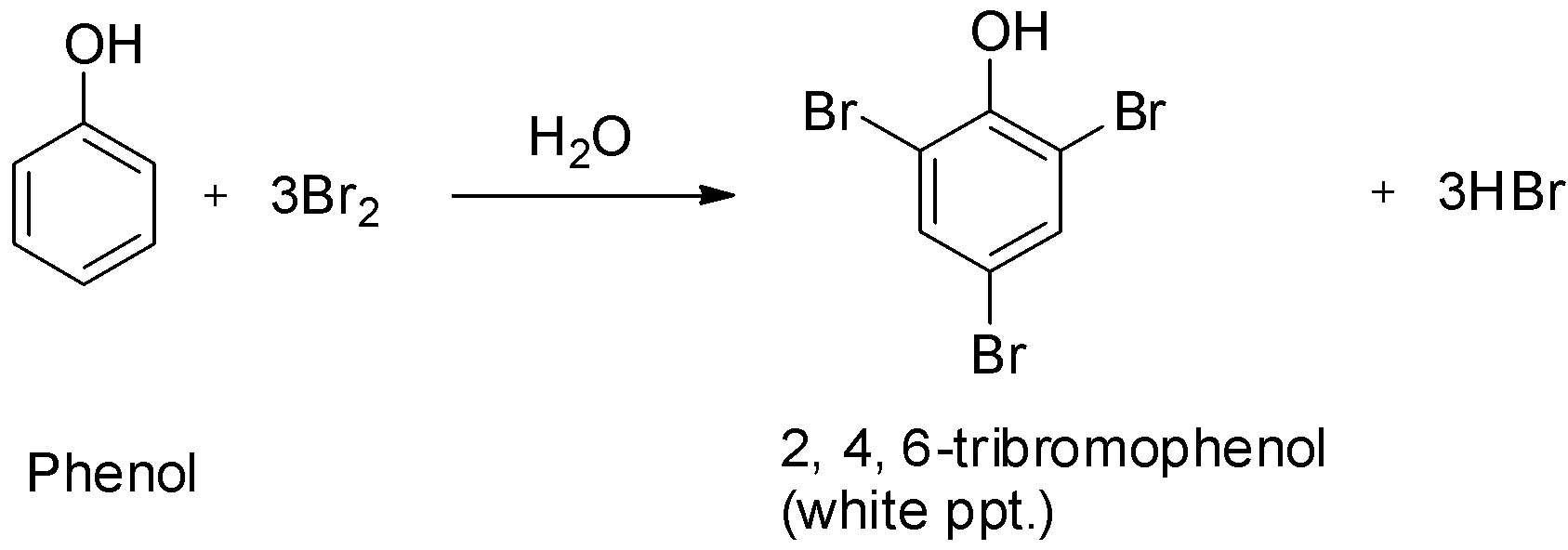
Phenols on treatment with bromine water give the corresponding polybrominated derivative in which all the hydrogen atoms present at the ortho and para positions with respect to the hydroxyl group are replaced by bromine atoms. The product formed is 2, 4, 6-tribromophenol.
Note:
Some other oxidizing agents for conversion of alcohols to carbonyl compounds are Corey’s reagent or PCC, PDC and Jone’s reagent.
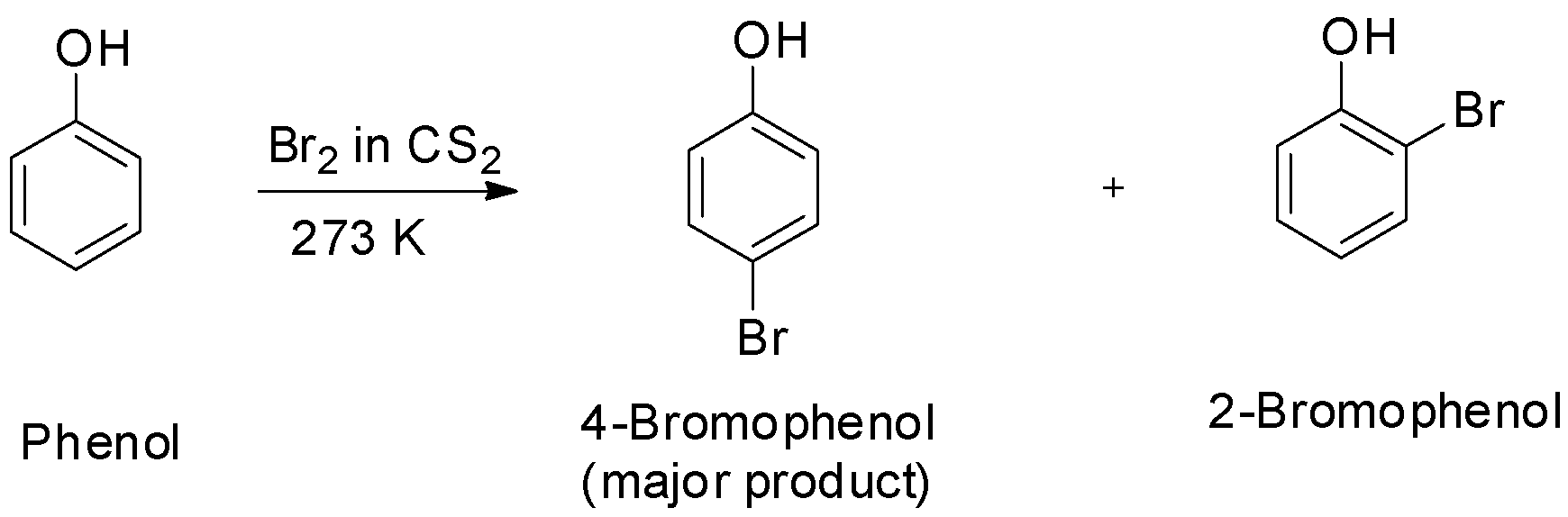
In presence of non-polar solvents, the ionization of phenol is suppressed due to which the oxygen of the hydroxyl group donates electrons to the ring only to a small extent. As a result, only little ring activation occurs and only monosubstitution occurs.
Complete step by step answer:
A.Conversion of propan-2-ol to propanone:
On controlled oxidation, alcohols give aldehydes or ketones. Primary alcohols on controlled oxidation give aldehydes, whereas secondary alcohols on controlled oxidation give ketones.
The common oxidizing agents which are used for the purpose of converting the alcohols to aldehydes or ketones are acidified potassium dichromate which has the chemical formula ${{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}$ , aqueous or alkaline potassium permanganate which has the chemical formula ${\text{KMn}}{{\text{O}}_{\text{4}}}$ and chromic anhydride or chromium trioxide ${\text{Cr}}{{\text{O}}_{\text{3}}}$ and sulphuric acid, ${{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$ .
The general reaction of conversion of a primary alcohol to its corresponding aldehyde by oxidation using acidified potassium dichromate as oxidizing agent is shown below:
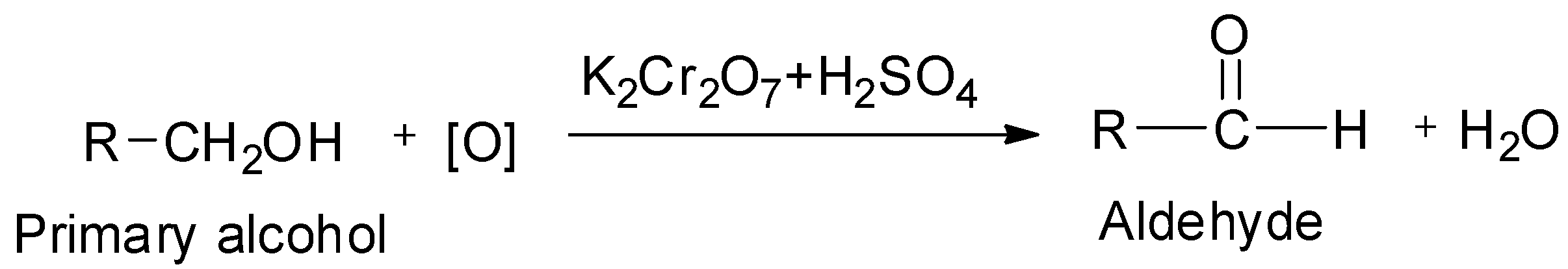
Now, propan-2-ol is a secondary alcohol as evident from its structure.

Therefore, propan-2-ol on oxidation with acidified potassium dichromate as an oxidizing agent will give its corresponding ketone. This ketone is propanone.
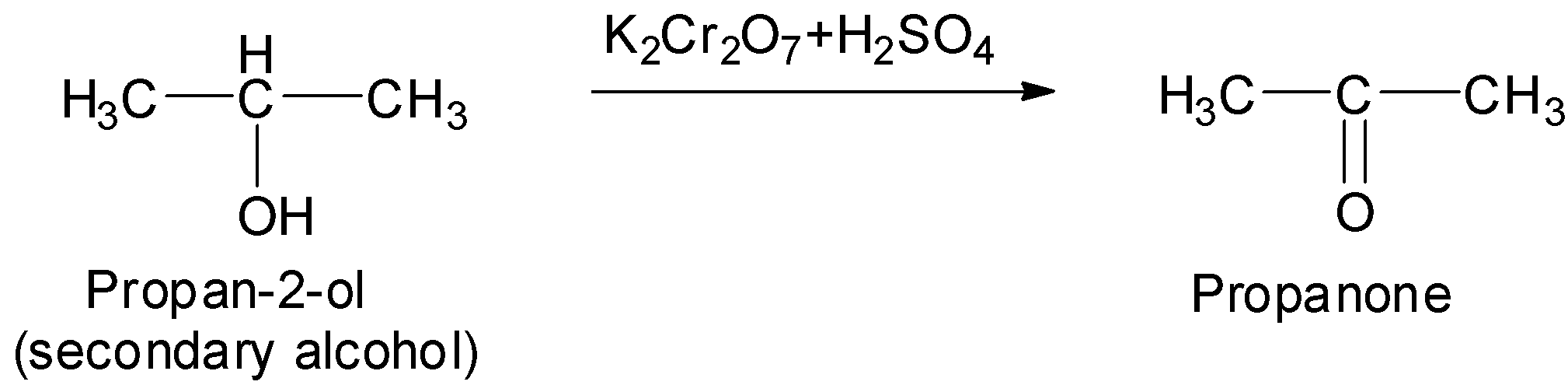
Thus, in this way we can convert propan-2-ol to propanone.
B.Conversion of phenol to 2, 4, 6-tribromophenol:
Due to the strong activating effect of the hydroxyl group attached to the benzene ring, halogenation of the benzene ring can take place even without a Lewis acid ultimately leading to polysubstitution. Moreover, the hydroxyl group is ortho, para directing and so it increases the electron density more at ortho and para positions.
Phenols on treatment with bromine water give the corresponding polybrominated derivative in which all the hydrogen atoms present at the ortho and para positions with respect to the hydroxyl group are replaced by bromine atoms. The product formed is 2, 4, 6-tribromophenol.

Note:
Some other oxidizing agents for conversion of alcohols to carbonyl compounds are Corey’s reagent or PCC, PDC and Jone’s reagent.
In presence of non-polar solvents, the ionization of phenol is suppressed due to which the oxygen of the hydroxyl group donates electrons to the ring only to a small extent. As a result, only little ring activation occurs and only monosubstitution occurs.

Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Give simple chemical tests to distinguish between the class 12 chemistry CBSE

Define Vant Hoff factor How is it related to the degree class 12 chemistry CBSE




