
How to convert chlorobenzene to $p - $nitrophenol?
Answer
503.7k+ views
Hint: To convert chlorobenzene to $p - $nitrophenol. We will first react to the chlorobenzene with sodium hydroxide and then it gives phenol. Then we will react to the phenol in the presence of concentrated $HN{O_3}$ and concentrated sulphuric acid. For further explanation of the process refer to the solution.
Complete answer:
This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals. The major use of chlorobenzene is as an intermediate in the production of commodities such as herbicides, dyestuffs, and rubber. Chlorobenzene is also used as a high-boiling solvent in many industrial applications as well as in the laboratory.
To convert chlorobenzene to $p - $ nitrophenol First the chlorine from Chlorobenzene is substituted by reacting with sodium hydroxide with an alcohol group. In this step phenol is formed. Then performing nitration to this intermediate product in presence of concentrated nitric acid and sulphuric acid to form $p - $nitrophenol.
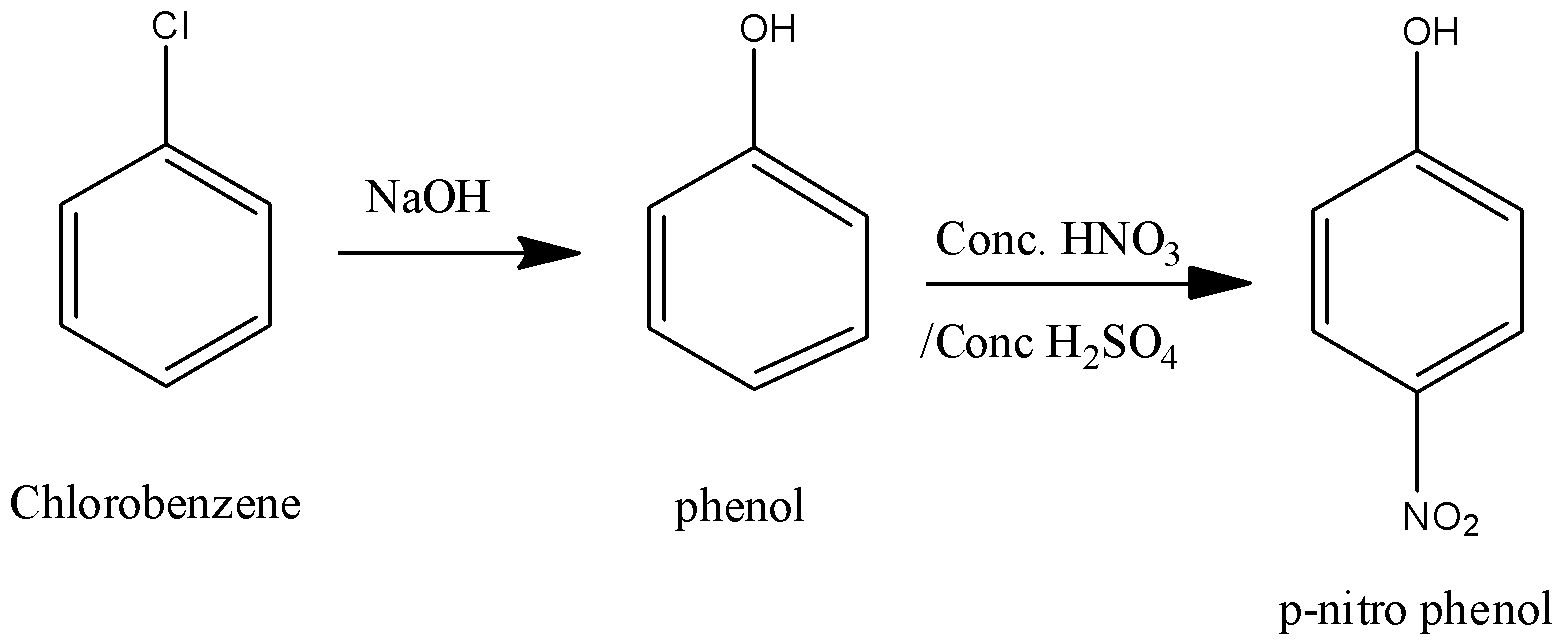
When phenol undergoes nitration it undergoes electrophilic substitution at ortho and para position because the lone pair on oxygen atom stabilizes the intermediate carbocation and the stabilization is maximum at ortho and para position. Thus the presence of hydroxyl group makes the ring activating and reactive at ortho and para position.
Note:
Direct nitration of phenol by dilute nitric acid gives modest yields of nitrated phenols and considerable oxidative decomposition to tarry materials aniline is largely destroyed. Nitration of phenol with dilute nitric acid gives the mixture of ortho-nitrophenol and para-nitrophenol. But the major product is ortho-nitrophenol. Since it is an electrophilic aromatic substitution reaction.
Complete answer:
This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals. The major use of chlorobenzene is as an intermediate in the production of commodities such as herbicides, dyestuffs, and rubber. Chlorobenzene is also used as a high-boiling solvent in many industrial applications as well as in the laboratory.
To convert chlorobenzene to $p - $ nitrophenol First the chlorine from Chlorobenzene is substituted by reacting with sodium hydroxide with an alcohol group. In this step phenol is formed. Then performing nitration to this intermediate product in presence of concentrated nitric acid and sulphuric acid to form $p - $nitrophenol.

When phenol undergoes nitration it undergoes electrophilic substitution at ortho and para position because the lone pair on oxygen atom stabilizes the intermediate carbocation and the stabilization is maximum at ortho and para position. Thus the presence of hydroxyl group makes the ring activating and reactive at ortho and para position.
Note:
Direct nitration of phenol by dilute nitric acid gives modest yields of nitrated phenols and considerable oxidative decomposition to tarry materials aniline is largely destroyed. Nitration of phenol with dilute nitric acid gives the mixture of ortho-nitrophenol and para-nitrophenol. But the major product is ortho-nitrophenol. Since it is an electrophilic aromatic substitution reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




