
Convert chlorobenzene to p- chloronitrobenzene.
Answer
567.3k+ views
Hint: The reaction of chlorobenzene to p- chloronitrobenzene is an electrophilic substitution reaction in which a nitro group is substituted on a benzene ring.
The first step would be the generation of electrophile i.e. nitronium ion. Then this nitronium ion will get substituted on the benzene ring.
Complete step by step answer :
The benzene is an aromatic compound. It is stabilised because of resonance. It does not undergo addition reactions because if it does, then the resonance of the ring is disturbed and it gets destabilised. Thus, it undergoes electrophilic substitution reaction.
The reaction of chlorobenzene to p- chloronitrobenzene is an electrophilic substitution reaction in which a nitro group is substituted on a benzene ring.
The electrophile is nitronium ion. The reaction takes place in two steps.
Step 1 : This step involves the generation of electrophile that is nitronium ion. The sulphuric acid and nitric acid react to form nitronium ion and some other products. The reaction is as-
$2{H_2}S{O_4} + HN{O_3} \to \oplus N{O_2} + 2HSO_4^ - + {H_3}O $
Step 2 : This step involves the attack of benzene on nitronium ion to give para products. The resonance is disturbed initially but later on after removal of proton, the resonance is restored. So, one gets a para-substituted product. The reaction is written as -
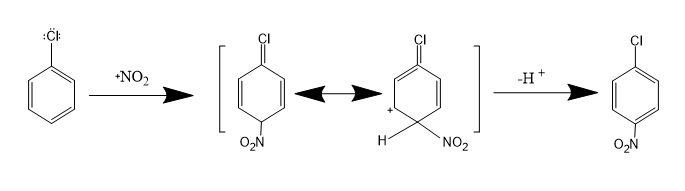
Note: The Chlorine is an ortho para directing group. This means that it directs the incoming group to ortho and para positions. The nitrogen atom in nitronium ion has positive charge. So, it will make it more reactive towards electrophilic substitution reaction.
The first step would be the generation of electrophile i.e. nitronium ion. Then this nitronium ion will get substituted on the benzene ring.
Complete step by step answer :
The benzene is an aromatic compound. It is stabilised because of resonance. It does not undergo addition reactions because if it does, then the resonance of the ring is disturbed and it gets destabilised. Thus, it undergoes electrophilic substitution reaction.
The reaction of chlorobenzene to p- chloronitrobenzene is an electrophilic substitution reaction in which a nitro group is substituted on a benzene ring.
The electrophile is nitronium ion. The reaction takes place in two steps.
Step 1 : This step involves the generation of electrophile that is nitronium ion. The sulphuric acid and nitric acid react to form nitronium ion and some other products. The reaction is as-
$2{H_2}S{O_4} + HN{O_3} \to \oplus N{O_2} + 2HSO_4^ - + {H_3}O $
Step 2 : This step involves the attack of benzene on nitronium ion to give para products. The resonance is disturbed initially but later on after removal of proton, the resonance is restored. So, one gets a para-substituted product. The reaction is written as -

Note: The Chlorine is an ortho para directing group. This means that it directs the incoming group to ortho and para positions. The nitrogen atom in nitronium ion has positive charge. So, it will make it more reactive towards electrophilic substitution reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




