
Concentrated \[{{NH}}{{{O}}_{{{3}}\,\,\,}}\] forms \[{{NO}}_{{2}}^{{ + }}\] in the presence of:
A. Con. \[{{{H}}_{{2}}}{{S}}{{{O}}_{{4}}}\]
B. \[{{Fe}}\]
C. \[{{FeB}}{{{r}}_{{{3}}\,}}\]
D. \[{{AlC}}{{{l}}_{{3}}}\]
Answer
558.6k+ views
Hint: \[{{HN}}{{{O}}_{{{3}}\,\,\,}}\] is a strong acid. It is also known by the spirit of niter and aqua fortis. It is colourless but as it gets older it turns into a yellow in colour. This is due to the decomposition of nitric acid into oxides of nitrogen and water.
Complete step by step answer:
It is used as a strong oxidizing agent. It is synthesised from ammonia by catalytic oxidation. It is a major chemical used in industries to manufacture explosives and fertilizers. The ${{pH}}$ of Nitric acid is around \[3.01\]. Structurally, this molecule contains three oxygen atoms, one nitrogen atom, and one hydrogen atom. Here, one of the oxygen atoms is doubly bonded with the central nitrogen atom. Another oxygen atom is bonded singly with the central nitrogen atom and the oxygen is also singly bonded with the hydrogen atom. The oxygen atom that is singly bonded to the central nitrogen atom has a charge of $ - 1$. The central nitrogen atom of the molecule is participating in four covalent bonds (with 3 oxygen atoms), it has a charge of $ + 1$. Thus, the net charge on the nitric acid molecule is zero. The charges here can be delocalized due to resonance. The structure of nitric acid molecules can be represented as,
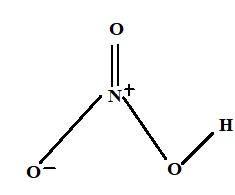
Concentrated nitric acid reacts with sulphuric acid to form \[{{NO}}_{{2}}^{{ + }}\], the equation is represented as,
\[{{O}}{{{H}}^{{ - }}}\,\,{{ + }}\,\,{{{N}}^{{ + }}}{{O}}_{{2}}^{}\,\,{{ + }}\,\,{{{H}}_{{2}}}{{S}}{{{O}}_{{4}}}\,\, \to \,{{{H}}_{{2}}}{{O}}\,\,{{ + }}\,\,{{{H}}_{{2}}}{{S}}{{{O}}_{{4}}}\,\]
So, the correct answer is Option A.
Additional Information:
It is highly corrosive and toxic. It causes severe skin burn. It reacts with hydroxides, metals, and oxides to form nitrate salts.
Note: Some of the application of nitric acid are:
It is used to manufacture dye, and fertilizers.
It can be used in manufacturing explosives such as TNT.
It is used as an oxidizer in liquid-fuelled rockets.
It is in electrochemistry as a chemical doping agent.
Complete step by step answer:
It is used as a strong oxidizing agent. It is synthesised from ammonia by catalytic oxidation. It is a major chemical used in industries to manufacture explosives and fertilizers. The ${{pH}}$ of Nitric acid is around \[3.01\]. Structurally, this molecule contains three oxygen atoms, one nitrogen atom, and one hydrogen atom. Here, one of the oxygen atoms is doubly bonded with the central nitrogen atom. Another oxygen atom is bonded singly with the central nitrogen atom and the oxygen is also singly bonded with the hydrogen atom. The oxygen atom that is singly bonded to the central nitrogen atom has a charge of $ - 1$. The central nitrogen atom of the molecule is participating in four covalent bonds (with 3 oxygen atoms), it has a charge of $ + 1$. Thus, the net charge on the nitric acid molecule is zero. The charges here can be delocalized due to resonance. The structure of nitric acid molecules can be represented as,

Concentrated nitric acid reacts with sulphuric acid to form \[{{NO}}_{{2}}^{{ + }}\], the equation is represented as,
\[{{O}}{{{H}}^{{ - }}}\,\,{{ + }}\,\,{{{N}}^{{ + }}}{{O}}_{{2}}^{}\,\,{{ + }}\,\,{{{H}}_{{2}}}{{S}}{{{O}}_{{4}}}\,\, \to \,{{{H}}_{{2}}}{{O}}\,\,{{ + }}\,\,{{{H}}_{{2}}}{{S}}{{{O}}_{{4}}}\,\]
So, the correct answer is Option A.
Additional Information:
It is highly corrosive and toxic. It causes severe skin burn. It reacts with hydroxides, metals, and oxides to form nitrate salts.
Note: Some of the application of nitric acid are:
It is used to manufacture dye, and fertilizers.
It can be used in manufacturing explosives such as TNT.
It is used as an oxidizer in liquid-fuelled rockets.
It is in electrochemistry as a chemical doping agent.
Recently Updated Pages
Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE




