
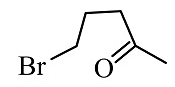
Complete the following reaction
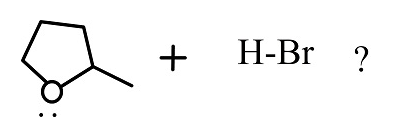
(1)
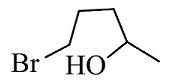
(2)
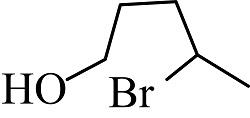
(3)
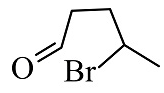
(4)
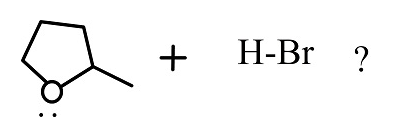




Answer
573.3k+ views
Hint: In this reaction ${S_N}2$ reaction mechanism takes place. This reaction involves a substitution of the leaving group which is generally a halide group with a nucleophilic compound. In this reaction the rate of reaction is determined by both the reactant species.
Complete step by step answer:
This reaction follows the ${S_N}2$ reaction mechanism which is a single step reaction. In this reaction substitution of a nucleophile in the organic compound takes. In reaction the nucleophile is bromine.
The reactants given are 2-methyltetrahydrofuran and hydrogen bromide. The lone pair of electrons present in the oxygen atom of 2-methyltetrahydrofuran abstract the hydrogen of the hydrogen bromide to form a cationic species and the nucleophile bromide ion is released. This state is the transition state where the bromide ion further attacks from backward direction to the positive charge and gets attached to the ring by forming a new bond in the chemical compound. The nucleophile reaches the chemical compound at the angle of 180 degree. In this mechanism, the breaking of bonds and formation of new bonds take place synchronously.
The reaction showing the ${S_N}2$reaction mechanism between 2-methyltetrahydrofuran and hydrogen bromide is shown below.
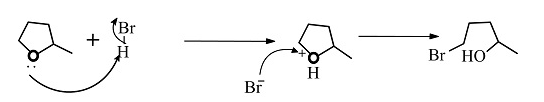
The second compound is in transition state.
So, the correct answer is Option 1.
Note: There are normally two ways by which the nucleophile can attack the carbon atom. First frontside attack and other is backside attack. As ${S_N}2$ reaction shows 100% inversion, it is confirmed that the nucleophile attacks the carbon atom from the backward direction. Primary substrate and secondary substrate can give ${S_N}2$ reaction mechanism but tertiary substrate cannot.
Complete step by step answer:
This reaction follows the ${S_N}2$ reaction mechanism which is a single step reaction. In this reaction substitution of a nucleophile in the organic compound takes. In reaction the nucleophile is bromine.
The reactants given are 2-methyltetrahydrofuran and hydrogen bromide. The lone pair of electrons present in the oxygen atom of 2-methyltetrahydrofuran abstract the hydrogen of the hydrogen bromide to form a cationic species and the nucleophile bromide ion is released. This state is the transition state where the bromide ion further attacks from backward direction to the positive charge and gets attached to the ring by forming a new bond in the chemical compound. The nucleophile reaches the chemical compound at the angle of 180 degree. In this mechanism, the breaking of bonds and formation of new bonds take place synchronously.
The reaction showing the ${S_N}2$reaction mechanism between 2-methyltetrahydrofuran and hydrogen bromide is shown below.

The second compound is in transition state.
So, the correct answer is Option 1.
Note: There are normally two ways by which the nucleophile can attack the carbon atom. First frontside attack and other is backside attack. As ${S_N}2$ reaction shows 100% inversion, it is confirmed that the nucleophile attacks the carbon atom from the backward direction. Primary substrate and secondary substrate can give ${S_N}2$ reaction mechanism but tertiary substrate cannot.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




