
Complete hydrolysis of starch gives:
A) glucose and galactose in equimolar amounts
B) galactose and fructose in equimolar amounts
C) glucose only
D) glucose and fructose in equimolar amount
Answer
569.7k+ views
Hint:Consider the monosaccharide units present in starch. Thy hydrolysis of a polysaccharide gives monosaccharides as the final product.
Complete answer:
We can represent starch with chemical formula \[{\left( {{{\text{C}}_6}{{\text{H}}_{10}}{{\text{O}}_5}} \right)_n}\] . Starch is a polymer or a polysaccharide. The monosaccharide unit present in starch is \[\alpha - D - \] glucopyranose unit.
Starch is insoluble in cold water, but We can prepare a colloidal solution of starch by grinding and soaking. On partial hydrolysis of starch and glycogen, We can obtain the disaccharide maltose and a low molecular weight dextran. Upon hydrolysis of starch We can get two fractions. The first fraction is around 20% of the hydrolyzed product. This fraction contains amylose that is water-soluble.
Amylose contains a linear chain that is made from several thousand glucose units. The second fraction that We obtain is called amylopectin. It contains around one million glucose units. Amylopectin is a branched polymer and is water-insoluble. Hence, when we subject starch to complete hydrolysis, We get \[\alpha - D - \]glucose as the sole product.
Let us write the structure of \[\alpha - D - \] glucopyranose as shown below:
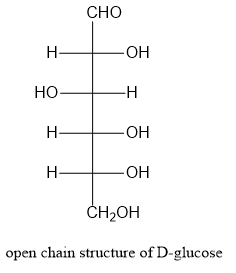
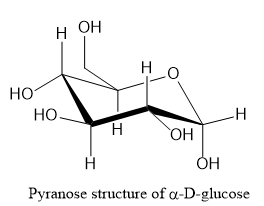
Note:Starch is a high molecular weight polysaccharide consisting of amylose and amylopectin. Amylose is a linear polymer and is around 20% of starch. Amylopectin is branched polymer and is around 80% of starch.
Complete answer:
We can represent starch with chemical formula \[{\left( {{{\text{C}}_6}{{\text{H}}_{10}}{{\text{O}}_5}} \right)_n}\] . Starch is a polymer or a polysaccharide. The monosaccharide unit present in starch is \[\alpha - D - \] glucopyranose unit.
Starch is insoluble in cold water, but We can prepare a colloidal solution of starch by grinding and soaking. On partial hydrolysis of starch and glycogen, We can obtain the disaccharide maltose and a low molecular weight dextran. Upon hydrolysis of starch We can get two fractions. The first fraction is around 20% of the hydrolyzed product. This fraction contains amylose that is water-soluble.
Amylose contains a linear chain that is made from several thousand glucose units. The second fraction that We obtain is called amylopectin. It contains around one million glucose units. Amylopectin is a branched polymer and is water-insoluble. Hence, when we subject starch to complete hydrolysis, We get \[\alpha - D - \]glucose as the sole product.
Let us write the structure of \[\alpha - D - \] glucopyranose as shown below:


Note:Starch is a high molecular weight polysaccharide consisting of amylose and amylopectin. Amylose is a linear polymer and is around 20% of starch. Amylopectin is branched polymer and is around 80% of starch.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




