
What is the common name of the compound shown in the image?
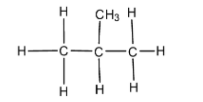
A) N-butane
B) Isobutane
C) Neobutane
D) Propane

Answer
573.9k+ views
Hint: The answer to the question lies in the concept of assigning names for the compound which is based on the chemical name it is called commonly which is accepted worldwide based on names given by the total number of carbon atoms present.
Complete answer:
We have studied the basic concepts of chemistry about the naming of given compounds on the basis of IUPAC rule and also about the common names assigned to it. These common names for the compound are based on the number of atoms present and specific names assigned to it. Now, for the above compound given in the question there are a total of four carbon atoms present among which only three are in the same contiguous form. Therefore, IUPAC name for this can be written according to the rules by numbering the carbon atom and writing it as 2 – methyl propane. But in this compound when written in simple form it appears as shown below,
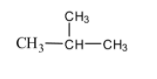
Now, since lower three carbon atoms are in continuous form and one methyl group is attached to the central carbon atom, the prefix given for this is ‘iso’ followed by the name for which the carbon atoms are four. Therefore, we can write the common name for this compound as isobutene.
Thus, the correct answer is option (B)
Note: Note that the IUPAC name includes the numerical in its name given and the common name does not have any numerical includes and can be spelled easily. This fact will be helpful for assigning the common names.
Complete answer:
We have studied the basic concepts of chemistry about the naming of given compounds on the basis of IUPAC rule and also about the common names assigned to it. These common names for the compound are based on the number of atoms present and specific names assigned to it. Now, for the above compound given in the question there are a total of four carbon atoms present among which only three are in the same contiguous form. Therefore, IUPAC name for this can be written according to the rules by numbering the carbon atom and writing it as 2 – methyl propane. But in this compound when written in simple form it appears as shown below,

Now, since lower three carbon atoms are in continuous form and one methyl group is attached to the central carbon atom, the prefix given for this is ‘iso’ followed by the name for which the carbon atoms are four. Therefore, we can write the common name for this compound as isobutene.
Thus, the correct answer is option (B)
Note: Note that the IUPAC name includes the numerical in its name given and the common name does not have any numerical includes and can be spelled easily. This fact will be helpful for assigning the common names.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




