
Colemanite is:
(a) ${ Na }_{ 2 }{ B }_{ 4 }{ O }_{ 7 }.10{ H }_{ 2 }O$
(b) ${ Ca }_{ 2 }{ B }_{ 6 }{ O }_{ 11 }.5{ H }_{ 2 }O$
(c) $Na{ BO }_{ 2 }$
(d) ${ H }_{ 3 }{ BO }_{ 3 }$
Answer
593.1k+ views
Hint: Colemanite is a borate which is used as an ore for the extraction of Boron, Boric acid and some borates. It is generally a transparent colourless crystal but it can also show different colours depending on its source location.
Complete answer:
Colemanite is a transparent/translucent crystal which could be colourless, white, yellow or even grey. It has a monoclinic crystal structure and was named after William Tell Coleman, founder of the California borax industry. It is Pyroelectric and piezoelectric at very low temperatures and is used as an ore to extract Boron. Colemanite is found in Paleogene and Neogene sediments in areas such as Kramer district, Daggett (California) and Death Valley.
Colemanite contains the oxides of calcium (CaO), Boron (${ B }_{ 2 }{ O }_{ 3 }$) along with some other oxides present in small percentages.
Colemanite is used as a glaze layer for pottery. But if the percentage of Colemanite is higher in the glaze, it may cause wrinkling of the fired surface which occurs due to the rapid decomposition of Colemanite when heated; this process is also called ‘decrepitation’.
There are many processes by which boric acid and borates can be isolated from Colemanite. We will describe one such method. Crude or calcined Colemanite is decomposed in the presence of carbon dioxide and water at a pressure greater than the atmospheric pressure and moderate temperature. This leads to the separation of the solid phase and the liquid phase. From the liquid phase boric acid is crystallized.
The formula for Colemanite is ${ Ca }_{ 2 }{ B }_{ 6 }{ O }_{ 11 }.5{ H }_{ 2 }O$ which is also written as $Ca[{ B }_{ 3 }{ O }_{ 4 }(OH{ ) }_{ 3 }].{ H }_{ 2 }O$. It chemical structure is given below:
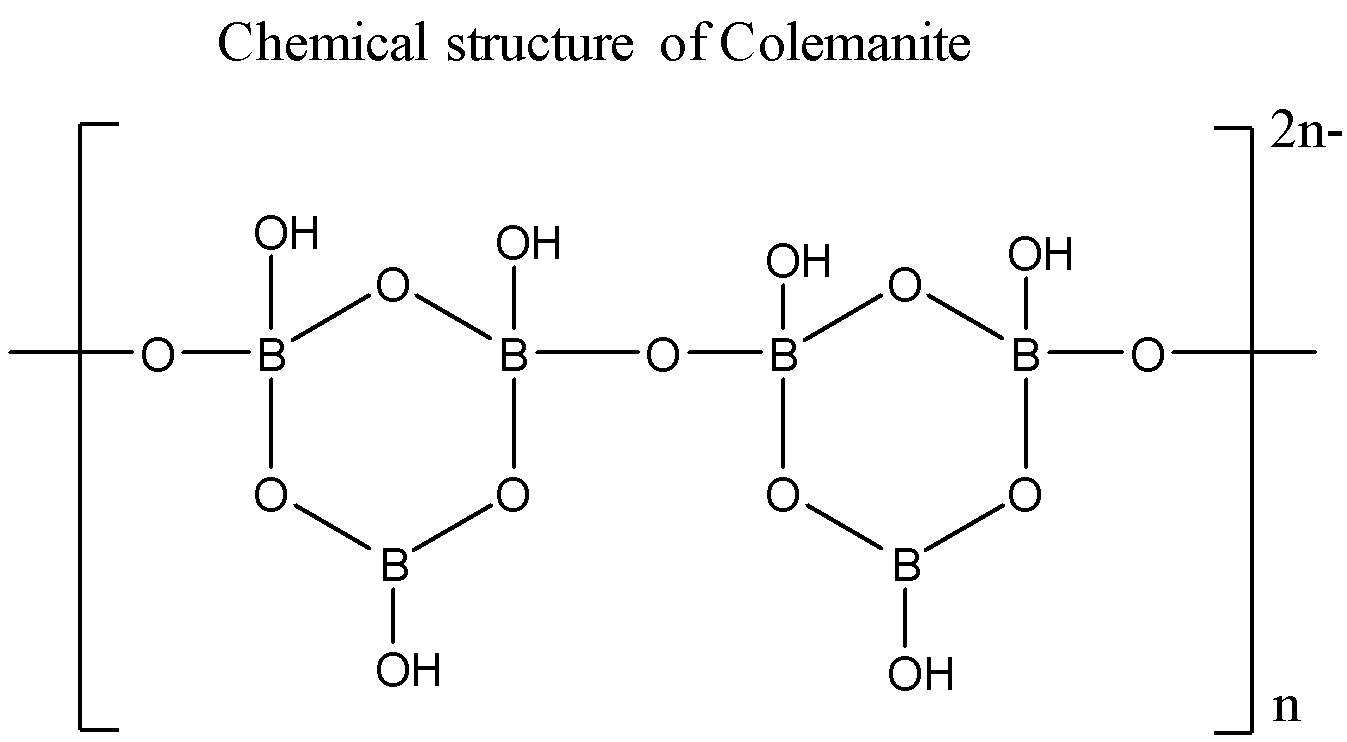
So, the correct answer is “Option (B) ${ Ca }_{ 2 }{ B }_{ 6 }{ O }_{ 11 }.5{ H }_{ 2 }O$.”
Note: Due to decrepitation it is better to avoid Colemanite for glazing. Instead of Colemanite, Gerstley Borate (it is a calcium borate containing Ulexite along with Colemanite, Bentonite and Probertite) and Ulexite ($NaCa{ B }_{ 5 }{ O }_{ 6 }(OH{ ) }_{ 6 }.5{ H }_{ 2 }O$) (both are similar minerals as Colemanite) can be used.
Complete answer:
Colemanite is a transparent/translucent crystal which could be colourless, white, yellow or even grey. It has a monoclinic crystal structure and was named after William Tell Coleman, founder of the California borax industry. It is Pyroelectric and piezoelectric at very low temperatures and is used as an ore to extract Boron. Colemanite is found in Paleogene and Neogene sediments in areas such as Kramer district, Daggett (California) and Death Valley.
Colemanite contains the oxides of calcium (CaO), Boron (${ B }_{ 2 }{ O }_{ 3 }$) along with some other oxides present in small percentages.
Colemanite is used as a glaze layer for pottery. But if the percentage of Colemanite is higher in the glaze, it may cause wrinkling of the fired surface which occurs due to the rapid decomposition of Colemanite when heated; this process is also called ‘decrepitation’.
There are many processes by which boric acid and borates can be isolated from Colemanite. We will describe one such method. Crude or calcined Colemanite is decomposed in the presence of carbon dioxide and water at a pressure greater than the atmospheric pressure and moderate temperature. This leads to the separation of the solid phase and the liquid phase. From the liquid phase boric acid is crystallized.
The formula for Colemanite is ${ Ca }_{ 2 }{ B }_{ 6 }{ O }_{ 11 }.5{ H }_{ 2 }O$ which is also written as $Ca[{ B }_{ 3 }{ O }_{ 4 }(OH{ ) }_{ 3 }].{ H }_{ 2 }O$. It chemical structure is given below:

So, the correct answer is “Option (B) ${ Ca }_{ 2 }{ B }_{ 6 }{ O }_{ 11 }.5{ H }_{ 2 }O$.”
Note: Due to decrepitation it is better to avoid Colemanite for glazing. Instead of Colemanite, Gerstley Borate (it is a calcium borate containing Ulexite along with Colemanite, Bentonite and Probertite) and Ulexite ($NaCa{ B }_{ 5 }{ O }_{ 6 }(OH{ ) }_{ 6 }.5{ H }_{ 2 }O$) (both are similar minerals as Colemanite) can be used.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




