
$C{{O}_{2}}$ and $Si{{O}_{2}}$ is a solid or gas?
(A) $C{{O}_{2}}$ gas and $Si{{O}_{2}}$ solid
(B) $C{{O}_{2}}$ solid and $Si{{O}_{2}}$ gas
(C) $C{{O}_{2}}$ gas and $Si{{O}_{2}}$ gas
Answer
573.6k+ views
Hint: Both carbon and silicon belong to the same group in the periodic table and this means that these atoms have similar properties. $C{{O}_{2}}$ consists of a carbon atom which is covalently double bonded to two oxygen atoms and $Si{{O}_{2}}$ or silicon dioxide is an oxide of silicon.
Complete answer:
-As we know, both carbon and silicon belong to the same group in the periodic table. Hence, both these atoms should have similar properties. These atoms have a tendency to show catenation which is the property of an element with which it can form a long chain by linking with other atoms of the same element.
- In carbon dioxide there is a double bond between the oxygen and carbon atoms. (O=C=O), each molecule is attracted to the other molecules through the London forces or van der waals forces.
- Because of the small atomic size, carbon atom possesses partial triple bond character with the neighboring oxygen atoms and due to the linear structure $C{{O}_{2}}$ is non-polar and hence they exhibit weak van der waals forces. As a result, Carbon dioxide ($C{{O}_{2}}$ ) exists as gas.
- In the case of $Si{{O}_{2}}$, every silicon atom is covalently bonded to four oxygen atoms and every oxygen atom is bonded to two silicon atoms. This will result in the formation of a giant tetrahedral structure which is shown below
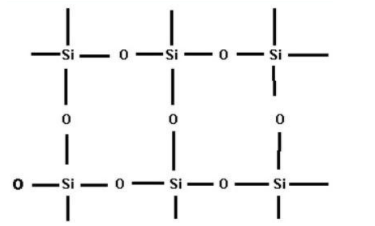
Because of this giant network, the bonding in $Si{{O}_{2}}$ is very strong and thus it exists as a solid. Therefore the $C{{O}_{2}}$ molecule exists as gas and $Si{{O}_{2}}$ exists as solid.
Thus the answer is option (A) $C{{O}_{2}}$ gas and $Si{{O}_{2}}$ solid.
Note: It should be noted that, $C{{O}_{2}}$ is an acidic oxide (forms carbonic acid on reaction with water) and $Si{{O}_{2}}$ doesn’t react with water, due to the difficulty of breaking up the giant covalent structure. Instead, $Si{{O}_{2}}$ is very weakly acidic when reacting with bases.
Complete answer:
-As we know, both carbon and silicon belong to the same group in the periodic table. Hence, both these atoms should have similar properties. These atoms have a tendency to show catenation which is the property of an element with which it can form a long chain by linking with other atoms of the same element.
- In carbon dioxide there is a double bond between the oxygen and carbon atoms. (O=C=O), each molecule is attracted to the other molecules through the London forces or van der waals forces.
- Because of the small atomic size, carbon atom possesses partial triple bond character with the neighboring oxygen atoms and due to the linear structure $C{{O}_{2}}$ is non-polar and hence they exhibit weak van der waals forces. As a result, Carbon dioxide ($C{{O}_{2}}$ ) exists as gas.
- In the case of $Si{{O}_{2}}$, every silicon atom is covalently bonded to four oxygen atoms and every oxygen atom is bonded to two silicon atoms. This will result in the formation of a giant tetrahedral structure which is shown below

Because of this giant network, the bonding in $Si{{O}_{2}}$ is very strong and thus it exists as a solid. Therefore the $C{{O}_{2}}$ molecule exists as gas and $Si{{O}_{2}}$ exists as solid.
Thus the answer is option (A) $C{{O}_{2}}$ gas and $Si{{O}_{2}}$ solid.
Note: It should be noted that, $C{{O}_{2}}$ is an acidic oxide (forms carbonic acid on reaction with water) and $Si{{O}_{2}}$ doesn’t react with water, due to the difficulty of breaking up the giant covalent structure. Instead, $Si{{O}_{2}}$ is very weakly acidic when reacting with bases.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




