
Classify $Na{{H}_{2}}P{{O}_{2}}$ and $NaHS{{O}_{4}}$ salts as acid salt or normal salt.
Answer
577.2k+ views
Hint: In oxoacids, the hydrogen atom that is attached to the oxygen atom causes the acidic nature of the oxoacids. So if the compound has more than one acidic hydrogen atom then the resulting salt will be acidic, and if the compound has only one acidic hydrogen atom then the resulting salt is normal or neutral.
Complete step by step answer:
-Both compounds given in the question are salts of oxoacids of respective elements. In $Na{{H}_{2}}P{{O}_{2}}$, phosphorus is the central atom, so it must be salt of oxoacids of phosphorus. In $NaHS{{O}_{4}}$, sulfur is the central atom, so it must be salt of oxoacids of sulfur.
-In oxoacids, the hydrogen atom that is attached to the oxygen atom causes the acidic nature of the oxoacids. So if the compound has more than one acidic hydrogen atom then the resulting salt will be acidic, and if the compound has only one acidic hydrogen atom then the resulting salt is normal or neutral.
-So the $Na{{H}_{2}}P{{O}_{2}}$, is the salt of ${{H}_{3}}P{{O}_{2}}$, there is only one acidic hydrogen atom is present as you can see in the diagram:
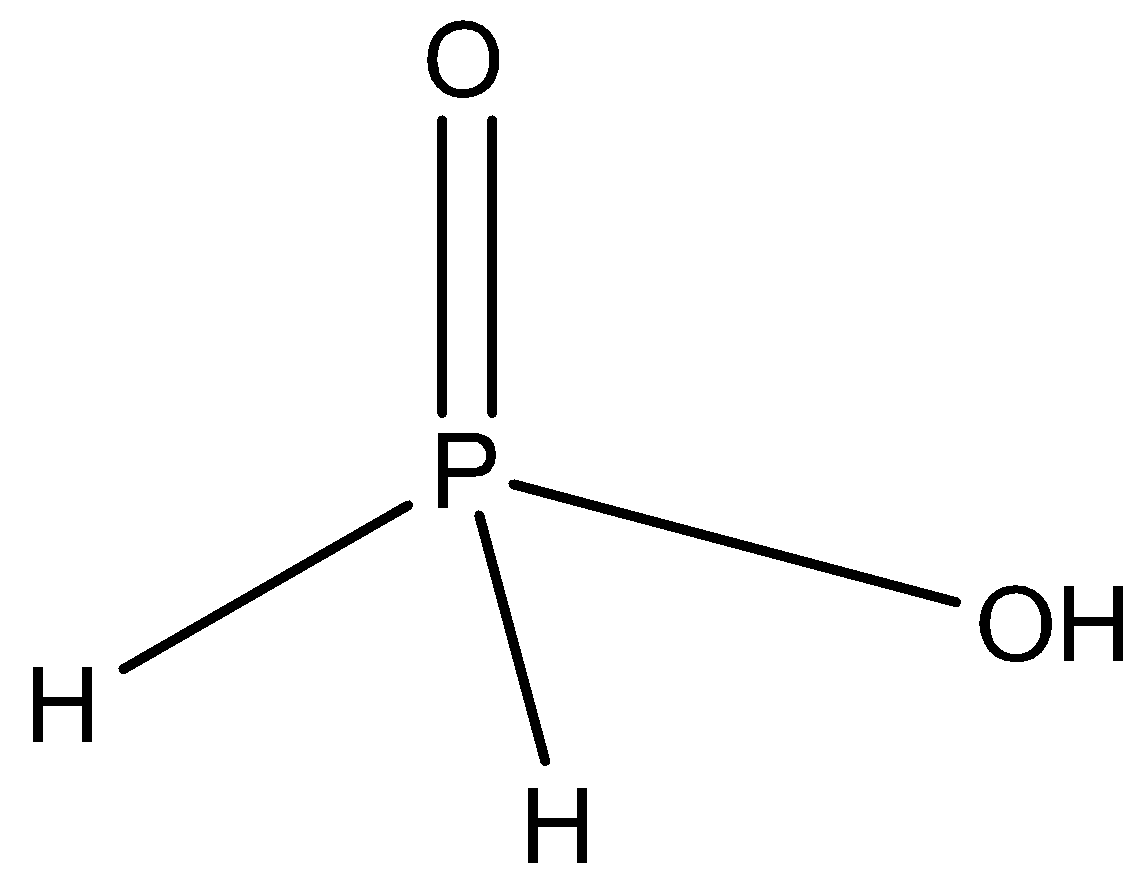
-So this hydrogen is substituted with sodium metal, so it is a normal or neutral salt.
-The $NaHS{{O}_{4}}$, is the salt of ${{H}_{2}}S{{O}_{4}}$, in ${{H}_{2}}S{{O}_{4}}$ there are two acidic hydrogen atoms are present as you can see in the diagram:
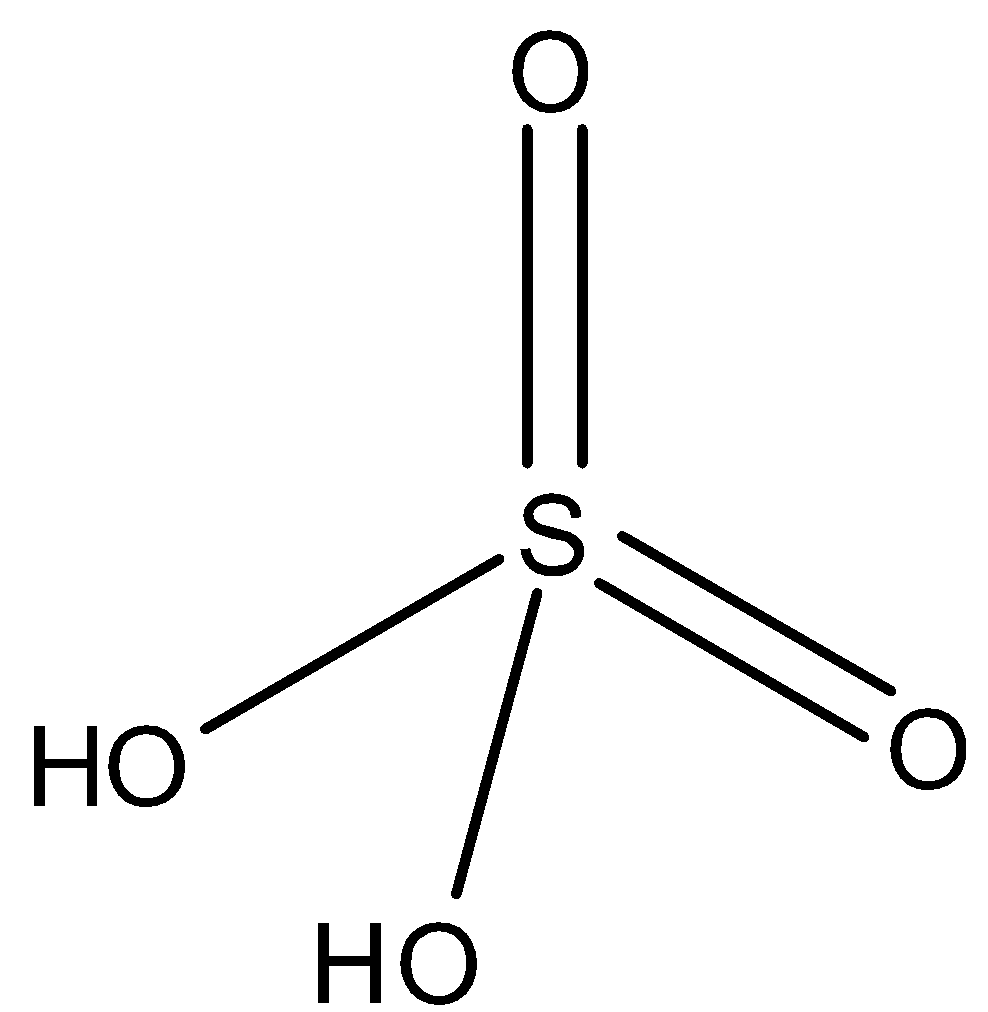
-So one hydrogen atom is substituted with sodium metal or partial substitution takes place, so it is an acid salt.
Note: The pH of the neutral salt or normal salt ranges from 5-7 and does not show any acidic or basic nature. Acid salt is always formed by incomplete replacement of hydrogen atoms.
Complete step by step answer:
-Both compounds given in the question are salts of oxoacids of respective elements. In $Na{{H}_{2}}P{{O}_{2}}$, phosphorus is the central atom, so it must be salt of oxoacids of phosphorus. In $NaHS{{O}_{4}}$, sulfur is the central atom, so it must be salt of oxoacids of sulfur.
-In oxoacids, the hydrogen atom that is attached to the oxygen atom causes the acidic nature of the oxoacids. So if the compound has more than one acidic hydrogen atom then the resulting salt will be acidic, and if the compound has only one acidic hydrogen atom then the resulting salt is normal or neutral.
-So the $Na{{H}_{2}}P{{O}_{2}}$, is the salt of ${{H}_{3}}P{{O}_{2}}$, there is only one acidic hydrogen atom is present as you can see in the diagram:

-So this hydrogen is substituted with sodium metal, so it is a normal or neutral salt.
-The $NaHS{{O}_{4}}$, is the salt of ${{H}_{2}}S{{O}_{4}}$, in ${{H}_{2}}S{{O}_{4}}$ there are two acidic hydrogen atoms are present as you can see in the diagram:

-So one hydrogen atom is substituted with sodium metal or partial substitution takes place, so it is an acid salt.
Note: The pH of the neutral salt or normal salt ranges from 5-7 and does not show any acidic or basic nature. Acid salt is always formed by incomplete replacement of hydrogen atoms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




