
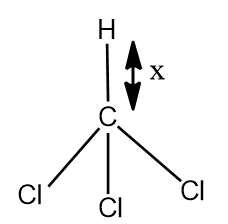
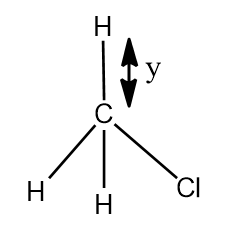
$CHC{{l}_{3}}$ and $C{{H}_{3}}Cl$ are given below, x and y are bond length of C-H bond in $CHC{{l}_{3}}$ and $C{{H}_{3}}Cl$ respectively. Select the correct statement:
A) x = y
B) $x \rangle y $
C) $y \rangle x $
D) Bond length cannot be compared.


Answer
574.8k+ views
Hint: The electronegativity for Cl is more than H.
- The greater the electronegativity, greater the pull of electrons.
Complete step by step answer:
- In the question two structures are given i.e. $CHC{{l}_{3}}$ and $C{{H}_{3}}Cl$, we have to say that the, which of the C-H bond given in the figure as x and y has the greater length.
- Bond length is the average distance between the nuclei of the bonded atom in the molecule.
- Bond length depends on many factors like electronegativity, bond order, radii of the bonded atom etc.
- Bond length is inversely proportional to the bond order, as bond order increases bond length decreases and the bond will be stronger.
But here the bond order is the same, so this parameter can’t be used here. C in both the compounds are $s{{p}^{3}}$ hybridized. So the s and p character is the same here.
- Now let’s see the atomic radii parameter, here we are supposed to say about the C - H bond in the structures. As the atomic radii of C atom and H atom will be the same in every element, so that can’t be considered.
- Now, we move on the next factor, i.e. electronegativity, with the aid of electronegativity we could explain the correct statement.
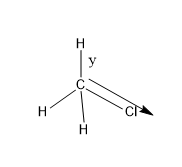
Let’s see both the structure:
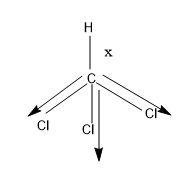
- In $CHC{{l}_{3}}$, there are three Cl atoms .The Cl atom is more electronegative than C hence the electrons shared between the bond will be will be attracted towards the Cl atom, and then bond will show polar nature whereas the C-H bond is non polar as the C and H atom does not show electronegative character.
- In $C{{H}_{3}}Cl$, there is only one Cl atom and that bond will show polar nature, and C-H bonds are nonpolar.
- As 3 electronegative atoms are present in the $CHC{{l}_{3}}$, it will results in the elongation of the C-H bond in $CHC{{l}_{3}}$ than in $C{{H}_{3}}Cl$
So, the correct answer is “Option B”.
Note: The C-Cl bond length also may be asked for a combination of options with aromatic compound and an aliphatic compound. In that case other factors like the atomic radii, the s and p character also come into effect.
- And the strength of C-H bond is greater than the C-Cl bond, since they break easily rather than the C-H bond and we get confused with this factor while calculating the bond length.
- Bond length should be calculated in considering all the effects that affect the bond length and the factor that overweights will be the deciding factor for the bond length.
- The greater the electronegativity, greater the pull of electrons.
Complete step by step answer:
- In the question two structures are given i.e. $CHC{{l}_{3}}$ and $C{{H}_{3}}Cl$, we have to say that the, which of the C-H bond given in the figure as x and y has the greater length.
- Bond length is the average distance between the nuclei of the bonded atom in the molecule.
- Bond length depends on many factors like electronegativity, bond order, radii of the bonded atom etc.
- Bond length is inversely proportional to the bond order, as bond order increases bond length decreases and the bond will be stronger.
But here the bond order is the same, so this parameter can’t be used here. C in both the compounds are $s{{p}^{3}}$ hybridized. So the s and p character is the same here.
- Now let’s see the atomic radii parameter, here we are supposed to say about the C - H bond in the structures. As the atomic radii of C atom and H atom will be the same in every element, so that can’t be considered.
- Now, we move on the next factor, i.e. electronegativity, with the aid of electronegativity we could explain the correct statement.
Let’s see both the structure:


- In $CHC{{l}_{3}}$, there are three Cl atoms .The Cl atom is more electronegative than C hence the electrons shared between the bond will be will be attracted towards the Cl atom, and then bond will show polar nature whereas the C-H bond is non polar as the C and H atom does not show electronegative character.
- In $C{{H}_{3}}Cl$, there is only one Cl atom and that bond will show polar nature, and C-H bonds are nonpolar.
- As 3 electronegative atoms are present in the $CHC{{l}_{3}}$, it will results in the elongation of the C-H bond in $CHC{{l}_{3}}$ than in $C{{H}_{3}}Cl$
So, the correct answer is “Option B”.
Note: The C-Cl bond length also may be asked for a combination of options with aromatic compound and an aliphatic compound. In that case other factors like the atomic radii, the s and p character also come into effect.
- And the strength of C-H bond is greater than the C-Cl bond, since they break easily rather than the C-H bond and we get confused with this factor while calculating the bond length.
- Bond length should be calculated in considering all the effects that affect the bond length and the factor that overweights will be the deciding factor for the bond length.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction

State the laws of reflection of light




