
Calculate total number of optical isomers in the following compounds:
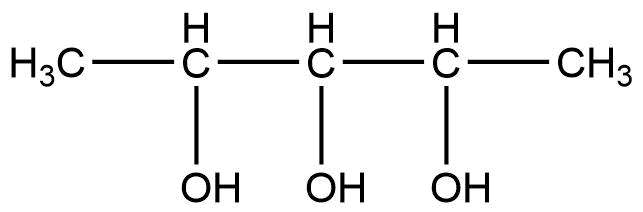
A \[{\text{2}}\]
B ${\text{4}}$
C ${\text{3}}$
D ${\text{8}}$

Answer
583.2k+ views
Hint: So here in this question we have to calculate the total number of optical isomers. Basically, optical isomers are two compounds which contain the same number and kinds of atoms, and bonds which have non – superimposable mirror images.
Complete step by step answer:
So in this compound there are 4 optical isomers and these are calculated when we multiply the number of chiral centers by two.
Mathematically, to calculate the total no. of optical isomers is ${{\text{2}}^{{\text{n - 1}}}}$, where n is equal to the number of chiral centers. So as in this compound there are three chiral carbons. So here we consider, ${\text{n = 3}}$
So by substituting the value of n, we get,
${\text{ = }}{{\text{2}}^{{\text{3 - 1}}}}$
${\text{ = }}{{\text{2}}^{\text{2}}}$
${\text{ = 4}}$
Additional Information: For optical isomers, molecules that are having chiral centers:-
If n is even, then the number of enantiomers ${\text{ = }}{{\text{2}}^{{\text{n - 1}}}}$
No. of meso compounds ${\text{ = }}{{\text{2}}^{{\text{n}} / {\text{2 - 1}}}}$
Total no. of optical isomers ${\text{ = }}{{\text{2}}^{{\text{n - 1}}}}{\text{ + }}{{\text{2}}^{{\text{n}} / {\text{2 - 1}}}}$
That is; number of enantiomers + number of meso compounds =Total no. of optical isomers.
If n is odd, then the number of enantiomers ${\text{ = }}{{\text{2}}^{{\text{n - 1}}}}{\text{ - }}{{\text{2}}^{\left( {{\text{n - 1}}} \right)}}^{ / {\text{2}}}$
No. of meso compounds ${\text{ = }}{{\text{2}}^{\left( {{\text{n - 1}}} \right) / {\text{2}}}}$
Total no. of optical isomers ${\text{ = }}{{\text{2}}^{{\text{n - 1}}}}$
So here, meso compounds are non-optically active members at least two of which are optically active.
And there are 2n optical isomers for an organic molecule with n chiral centers.
So, the correct answer is Option B .
Note:
Working on carbons is chiral, so we just have to check each one separately. A chiral carbon is not a branched group. A chiral carbon is a carbon atom that is bonded to four different atoms.
Complete step by step answer:
So in this compound there are 4 optical isomers and these are calculated when we multiply the number of chiral centers by two.
Mathematically, to calculate the total no. of optical isomers is ${{\text{2}}^{{\text{n - 1}}}}$, where n is equal to the number of chiral centers. So as in this compound there are three chiral carbons. So here we consider, ${\text{n = 3}}$
So by substituting the value of n, we get,
${\text{ = }}{{\text{2}}^{{\text{3 - 1}}}}$
${\text{ = }}{{\text{2}}^{\text{2}}}$
${\text{ = 4}}$
Additional Information: For optical isomers, molecules that are having chiral centers:-
If n is even, then the number of enantiomers ${\text{ = }}{{\text{2}}^{{\text{n - 1}}}}$
No. of meso compounds ${\text{ = }}{{\text{2}}^{{\text{n}} / {\text{2 - 1}}}}$
Total no. of optical isomers ${\text{ = }}{{\text{2}}^{{\text{n - 1}}}}{\text{ + }}{{\text{2}}^{{\text{n}} / {\text{2 - 1}}}}$
That is; number of enantiomers + number of meso compounds =Total no. of optical isomers.
If n is odd, then the number of enantiomers ${\text{ = }}{{\text{2}}^{{\text{n - 1}}}}{\text{ - }}{{\text{2}}^{\left( {{\text{n - 1}}} \right)}}^{ / {\text{2}}}$
No. of meso compounds ${\text{ = }}{{\text{2}}^{\left( {{\text{n - 1}}} \right) / {\text{2}}}}$
Total no. of optical isomers ${\text{ = }}{{\text{2}}^{{\text{n - 1}}}}$
So here, meso compounds are non-optically active members at least two of which are optically active.
And there are 2n optical isomers for an organic molecule with n chiral centers.
So, the correct answer is Option B .
Note:
Working on carbons is chiral, so we just have to check each one separately. A chiral carbon is not a branched group. A chiral carbon is a carbon atom that is bonded to four different atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




