
Calculate the % s-character in the lone pair electron of ${\rm{N}}{{\rm{H}}_{\rm{3}}}$, where the percentage s-character of bond pair is $22\% $ in each ${\rm{N - H}}$ bond.
A. $60\% $
B. $25\% $
C. $44\% $
D. $34\% $
Answer
583.2k+ views
Hint: We know that hybridization is generally explained by the overlapping of two or more atomic orbitals and they usually form new atomic orbitals through the pairing of electrons.
Complete step by step answer:
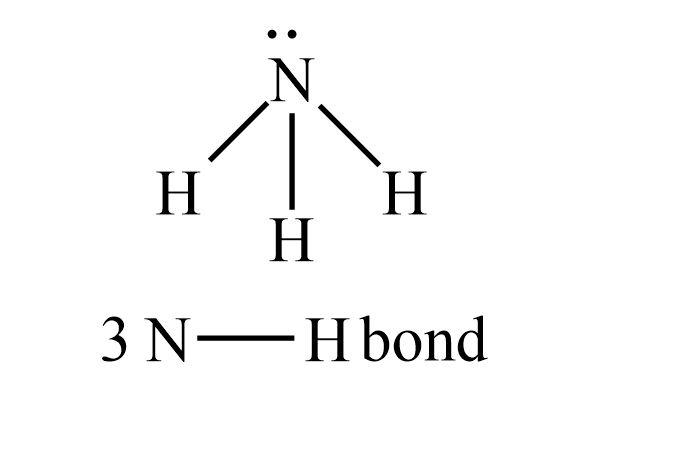
We know that, the shape of ${\rm{N}}{{\rm{H}}_{\rm{3}}}$ molecule is distorted tetrahedral and the hybridization of this molecule is ${\rm{s}}{{\rm{p}}^{\rm{3}}}$. In the ${\rm{N}}{{\rm{H}}_{\rm{3}}}$ molecule the number of ${\rm{N - H}}$ bond is three and it has one lone pair. The lone pair electrons are those electrons which are generally not shared with any other atom, and sometimes lone pair is termed as unshared electrons or non-bonding electrons.
The number of ${\rm{N - H}}$ bond present in ${\rm{N}}{{\rm{H}}_{\rm{3}}}$ molecule is three. So, the percentage of one bond pair showing s-character is $22\% $, then three bonds showing s-character can be calculated as shown below.
$\begin{array}{c}
1\;{\rm{N - H}} = 22\% \\
3\;{\rm{N - H}} = 22\% \times 3\\
= 66\%
\end{array}$
The % of s-character in the lone pair of ${\rm{N}}{{\rm{H}}_{\rm{3}}}$ molecule is $100\% - 66\% = 34\% $.
Thus, the % s-character in the lone pair of ${\rm{N}}{{\rm{H}}_{\rm{3}}}$ molecule is $34\% $, when the percentage of s-character due to ${\rm{N - H}}$ bond is $22\% $.
Hence, the correct choice for this question is D that is $34\% $.
Note:
The hybridization of ${\rm{N}}{{\rm{H}}_{\rm{3}}}$ molecule is ${\rm{s}}{{\rm{p}}^{\rm{3}}}$ and the shape is distorted tetrahedral. So, the percentage character of any orbital can be determined with the help of shape and angle.
Complete step by step answer:
We know that, the shape of ${\rm{N}}{{\rm{H}}_{\rm{3}}}$ molecule is distorted tetrahedral and the hybridization of this molecule is ${\rm{s}}{{\rm{p}}^{\rm{3}}}$. In the ${\rm{N}}{{\rm{H}}_{\rm{3}}}$ molecule the number of ${\rm{N - H}}$ bond is three and it has one lone pair. The lone pair electrons are those electrons which are generally not shared with any other atom, and sometimes lone pair is termed as unshared electrons or non-bonding electrons.
The number of ${\rm{N - H}}$ bond present in ${\rm{N}}{{\rm{H}}_{\rm{3}}}$ molecule is three. So, the percentage of one bond pair showing s-character is $22\% $, then three bonds showing s-character can be calculated as shown below.
$\begin{array}{c}
1\;{\rm{N - H}} = 22\% \\
3\;{\rm{N - H}} = 22\% \times 3\\
= 66\%
\end{array}$
The % of s-character in the lone pair of ${\rm{N}}{{\rm{H}}_{\rm{3}}}$ molecule is $100\% - 66\% = 34\% $.
Thus, the % s-character in the lone pair of ${\rm{N}}{{\rm{H}}_{\rm{3}}}$ molecule is $34\% $, when the percentage of s-character due to ${\rm{N - H}}$ bond is $22\% $.
Hence, the correct choice for this question is D that is $34\% $.
Note:
The hybridization of ${\rm{N}}{{\rm{H}}_{\rm{3}}}$ molecule is ${\rm{s}}{{\rm{p}}^{\rm{3}}}$ and the shape is distorted tetrahedral. So, the percentage character of any orbital can be determined with the help of shape and angle.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




