
Butylated hydroxyanisole is:
A. an antioxidant
B. cleansing agent
C. disinfectant
D. an antihistamine
Answer
590.7k+ views
Hint: Think about the nature and structure of butylated hydroxyanisole and its isomeric compounds that are present. Consider the properties of this molecule before answering the question. Also, consider similarities with butylated hydroxytoluene.
Complete step by step solution:
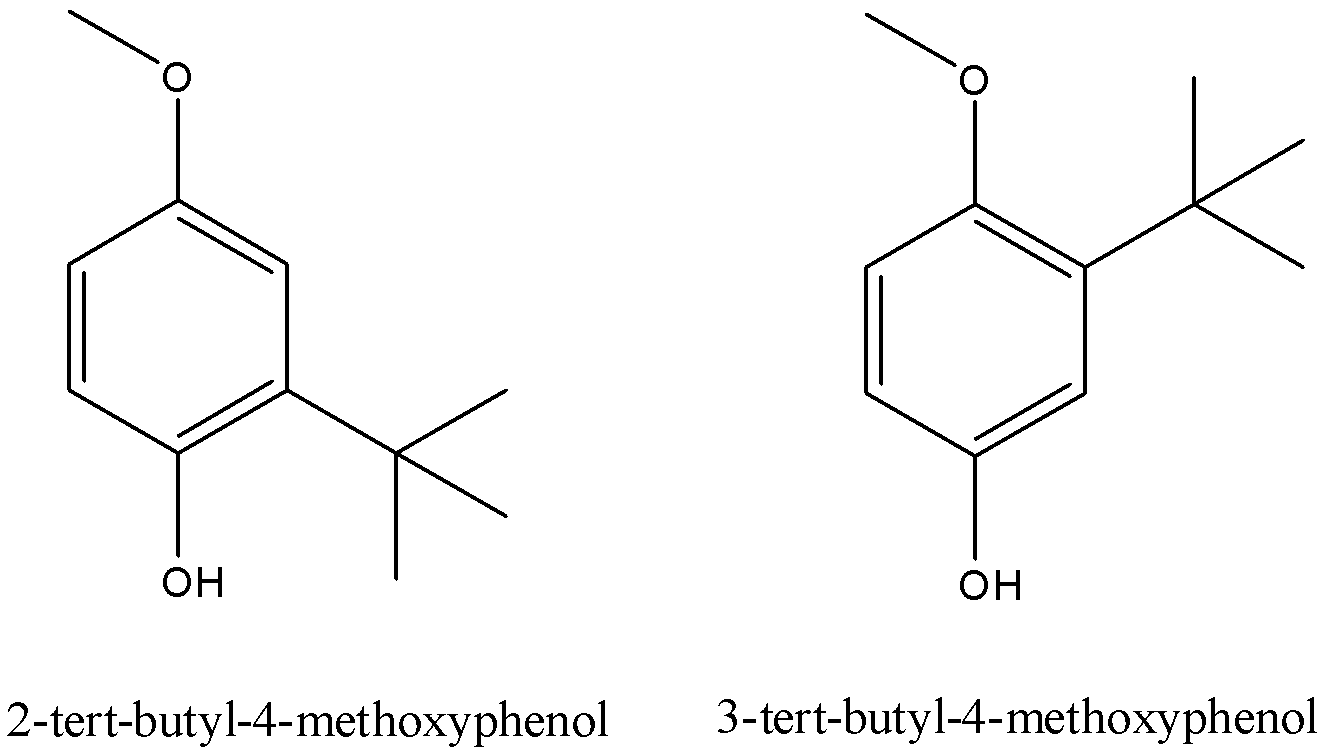
This butylated hydroxyanisole is present as a mixture of two isomeric molecules. Their IUPAC names are as follows: 2-tert-butyl-4-methoxyphenol and 3-tert-butyl-4-methoxyphenol. Their structures are as follows:
This butylated hydroxyanisole along with butylated hydroxytoluene has been added to the edible foodstuff that contains fats. These chemicals prevent the oxidation and rancidification of these fats and abolish any foul odour that may arise due to rancidification.
They have aromatic resonating rings that sequester any free radicals that may be present in the edible oils and fats and keep them in conjugation, thus preventing the oxidation of the fats.
Hence, the correct answer to this question is A. an antioxidant’
Additional information:
Cleansing agents consist of various soaps, detergents, abrasives, etc. They mostly include surfactants that have hydrophilic and hydrophobic ends that cleanse dirt and oil from surfaces.
Examples of disinfectants include chemicals like hydrogen peroxide, formaldehyde, glutaraldehydes, alcohols, etc.
Antihistamines are given as a medicine to allergic reactions by the body. Some examples include chlorpheniramine, loratadine, diphenhydramine, etc.
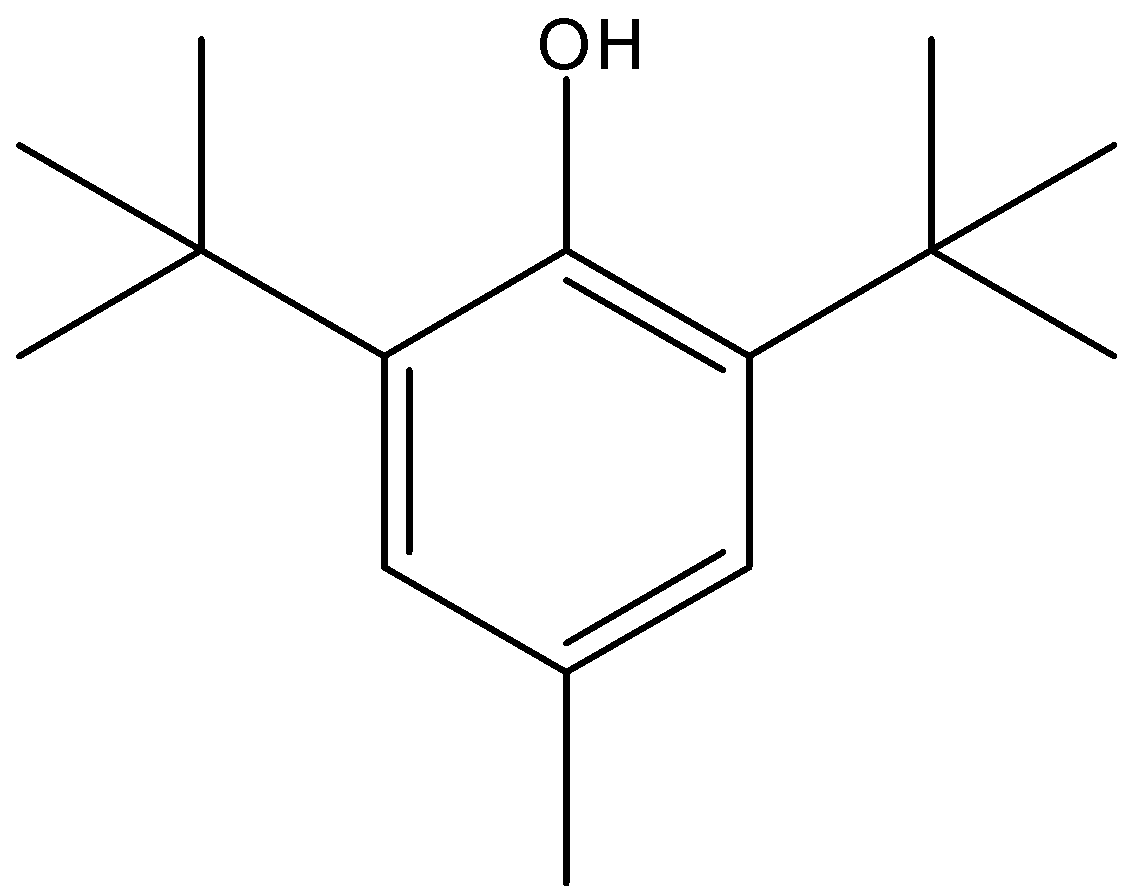
Note: Butylated hydroxytoluene (BHT) has also been used as an antioxidant along with its counterpart, butylated hydroxyanisole (BHA). BHT is also used to treat cold sores, genital herpes, and some symptoms of AIDS (acquired immunodeficiency syndrome). The structure of BHT is:
Complete step by step solution:
This butylated hydroxyanisole is present as a mixture of two isomeric molecules. Their IUPAC names are as follows: 2-tert-butyl-4-methoxyphenol and 3-tert-butyl-4-methoxyphenol. Their structures are as follows:

This butylated hydroxyanisole along with butylated hydroxytoluene has been added to the edible foodstuff that contains fats. These chemicals prevent the oxidation and rancidification of these fats and abolish any foul odour that may arise due to rancidification.
They have aromatic resonating rings that sequester any free radicals that may be present in the edible oils and fats and keep them in conjugation, thus preventing the oxidation of the fats.
Hence, the correct answer to this question is A. an antioxidant’
Additional information:
Cleansing agents consist of various soaps, detergents, abrasives, etc. They mostly include surfactants that have hydrophilic and hydrophobic ends that cleanse dirt and oil from surfaces.
Examples of disinfectants include chemicals like hydrogen peroxide, formaldehyde, glutaraldehydes, alcohols, etc.
Antihistamines are given as a medicine to allergic reactions by the body. Some examples include chlorpheniramine, loratadine, diphenhydramine, etc.
Note: Butylated hydroxytoluene (BHT) has also been used as an antioxidant along with its counterpart, butylated hydroxyanisole (BHA). BHT is also used to treat cold sores, genital herpes, and some symptoms of AIDS (acquired immunodeficiency syndrome). The structure of BHT is:

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




