
Both potassium ferrocyanide and potassium ferricyanide are diamagnetic.
A.True
B.False
Answer
566.7k+ views
Hint:We know that diamagnetism is the phenomenon in which an atom contains paired electrons and paramagnetism is the phenomenon in which an atom contains unpaired electrons.
Complete step by step answer:
The formula of potassium ferrocyanide is ${{\text{K}}_{\text{4}}}\left[ {{\text{Fe}}{{\left( {{\text{CN}}} \right)}_{\text{6}}}} \right]$. Now, we have to calculate the oxidation state of ${{\text{K}}_{\text{4}}}\left[ {{\text{Fe}}{{\left( {{\text{CN}}} \right)}_{\text{6}}}} \right]$.
We know that the oxidation state of potassium is +1 and cyanide is -1. Let’s take x as the oxidation state of Fe.
$x - 6 = - 4$
$ \Rightarrow x = 2$
So, the oxidation state of Fe is +2.
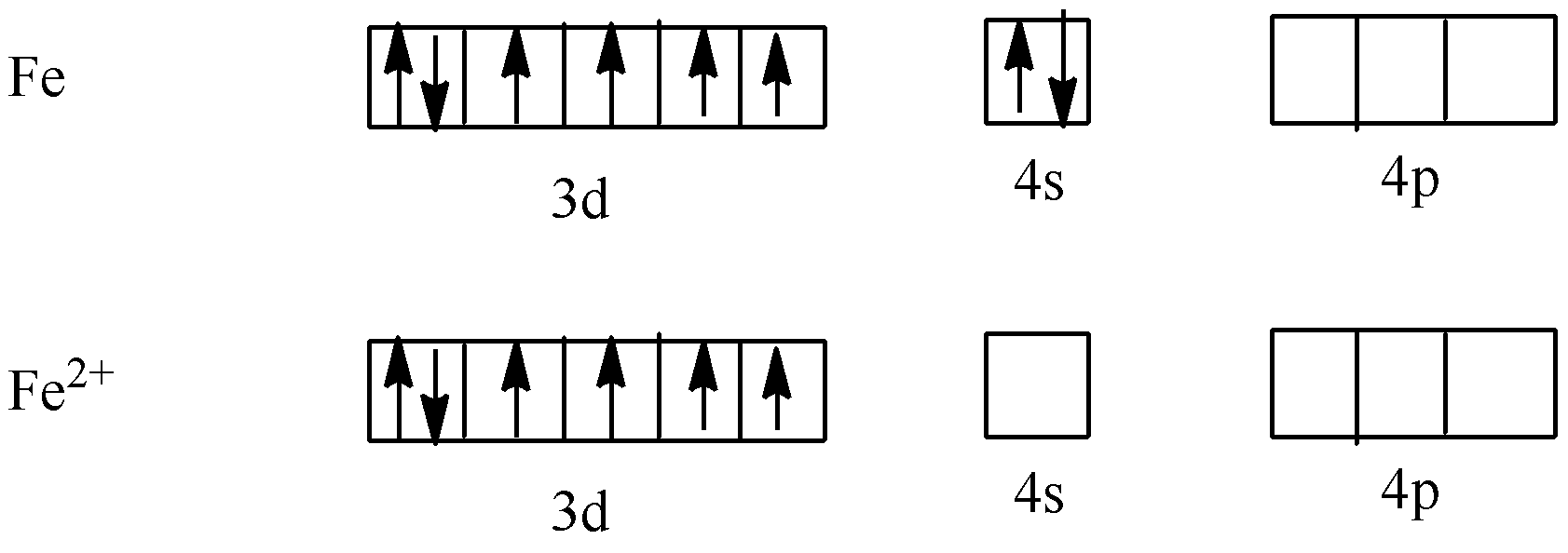
As CN is a strong field ligand. So, it causes the pairing of electrons. So,
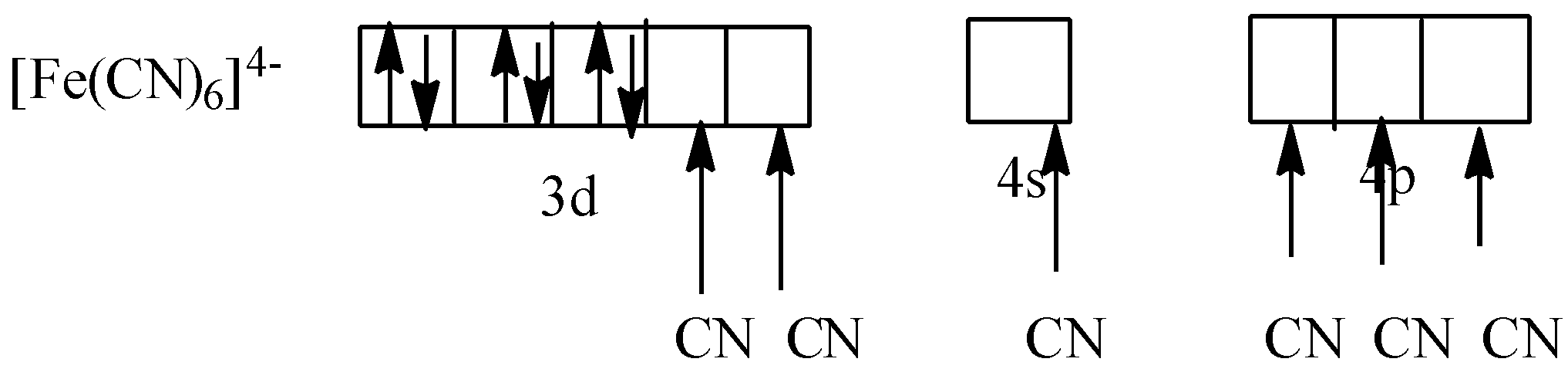
As all electrons are paired, potassium ferrocyanide is diamagnetic in nature.
Similarly we have to find out the magnetic character of potassium ferricyanide.
The formula of potassium ferricyanide is ${{\text{K}}_3}\left[ {{\text{Fe}}{{\left( {{\text{CN}}} \right)}_{\text{6}}}} \right]$. Now, we have to calculate the oxidation state of ${{\text{K}}_3}\left[ {{\text{Fe}}{{\left( {{\text{CN}}} \right)}_{\text{6}}}} \right]$.
We know that the oxidation state of potassium is +1 and cyanide is -1. Let’s take x as the oxidation state of Fe.
$x - 6 = - 3$
$ \Rightarrow x = 3$
So, the oxidation state of Fe is +3.
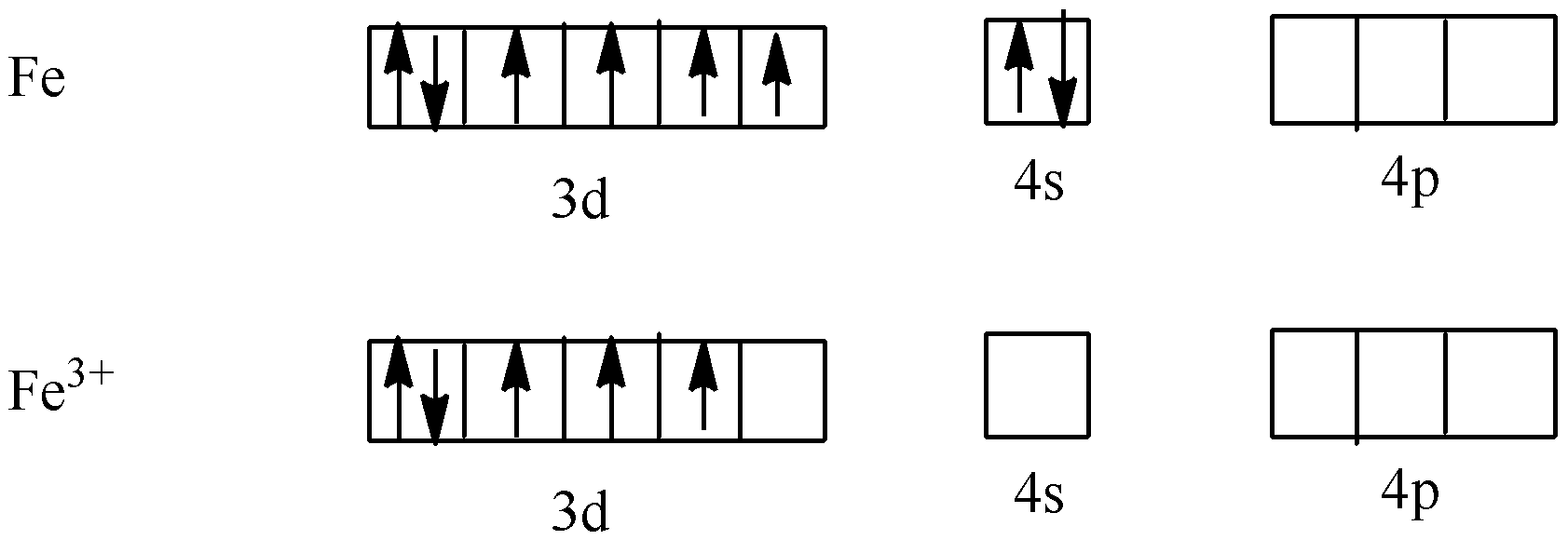
As CN is a strong field ligand. So, it causes the pairing of electrons. So,
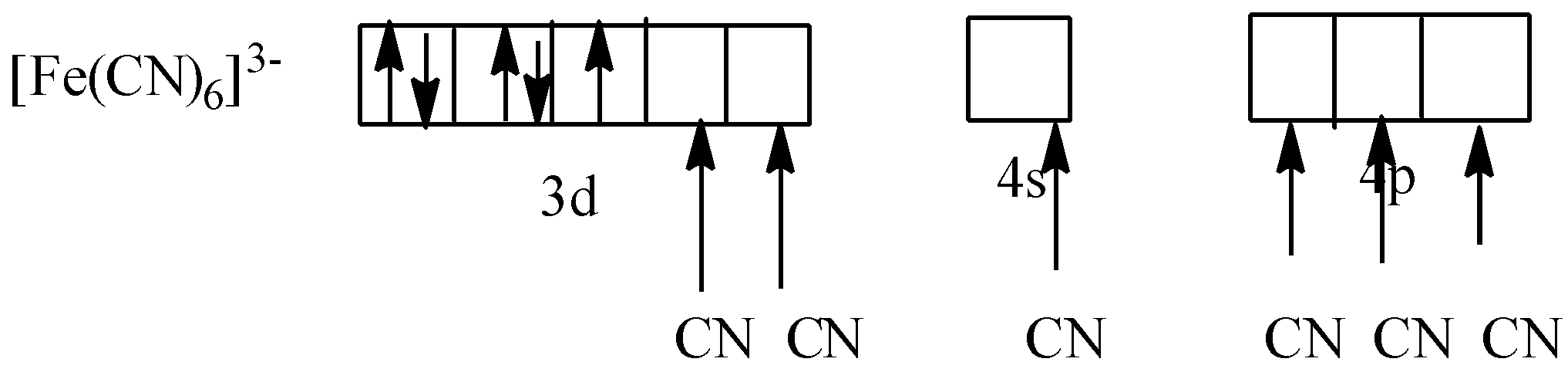
As one electron is not paired, so potassium ferricyanide is paramagnetic in nature.
Therefore, only potassium ferrocyanide is diamagnetic in nature.
Hence, the statement is false.
Note:
It is to be noted that there are two types of complexes in coordination compounds namely high spin complex and low spin complex. If the inner d orbital is used for the formation of compound then the compound is low spin complex ( ${d^2}s{p^3}$) and if the outer d orbital is used then the compound is high spin complex such as $s{p^3}{d^2}$.
Complete step by step answer:
The formula of potassium ferrocyanide is ${{\text{K}}_{\text{4}}}\left[ {{\text{Fe}}{{\left( {{\text{CN}}} \right)}_{\text{6}}}} \right]$. Now, we have to calculate the oxidation state of ${{\text{K}}_{\text{4}}}\left[ {{\text{Fe}}{{\left( {{\text{CN}}} \right)}_{\text{6}}}} \right]$.
We know that the oxidation state of potassium is +1 and cyanide is -1. Let’s take x as the oxidation state of Fe.
$x - 6 = - 4$
$ \Rightarrow x = 2$
So, the oxidation state of Fe is +2.

As CN is a strong field ligand. So, it causes the pairing of electrons. So,

As all electrons are paired, potassium ferrocyanide is diamagnetic in nature.
Similarly we have to find out the magnetic character of potassium ferricyanide.
The formula of potassium ferricyanide is ${{\text{K}}_3}\left[ {{\text{Fe}}{{\left( {{\text{CN}}} \right)}_{\text{6}}}} \right]$. Now, we have to calculate the oxidation state of ${{\text{K}}_3}\left[ {{\text{Fe}}{{\left( {{\text{CN}}} \right)}_{\text{6}}}} \right]$.
We know that the oxidation state of potassium is +1 and cyanide is -1. Let’s take x as the oxidation state of Fe.
$x - 6 = - 3$
$ \Rightarrow x = 3$
So, the oxidation state of Fe is +3.

As CN is a strong field ligand. So, it causes the pairing of electrons. So,

As one electron is not paired, so potassium ferricyanide is paramagnetic in nature.
Therefore, only potassium ferrocyanide is diamagnetic in nature.
Hence, the statement is false.
Note:
It is to be noted that there are two types of complexes in coordination compounds namely high spin complex and low spin complex. If the inner d orbital is used for the formation of compound then the compound is low spin complex ( ${d^2}s{p^3}$) and if the outer d orbital is used then the compound is high spin complex such as $s{p^3}{d^2}$.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




