
Boric acid has a polymeric layer structure in which planar $B{{O}_{3}}$ units are joined by:
(A)- covalent bonds
(B)- two centre-two electron bonds
(C)- coordinate bonds
(D)- hydrogen bonds
Answer
593.1k+ views
Hint: Boric acid (${{H}_{3}}B{{O}_{3}}$) in solid crystalline state has two dimensional layered structure. Hydrogen bond is a weak bond between an electronegative atom like N, O, F and a hydrogen atom bonded to another electronegative atom.
Complete answer:
To understand the structure of boric acid (${{H}_{3}}B{{O}_{3}}$), consider the ground state electronic configuration of boron.
B (in ground state): $1{{s}^{2}}2{{s}^{2}}2{{p}^{1}}$
One electron from 2s moves to 2p-orbital in the excited state and the electronic configuration becomes: $1{{s}^{2}}2{{s}^{1}}2{{p}^{2}}$
One s and two p-orbitals are now available to bond with three oxygen atoms. Therefore, the hybridization of the central atom B is$s{{p}^{2}}$.
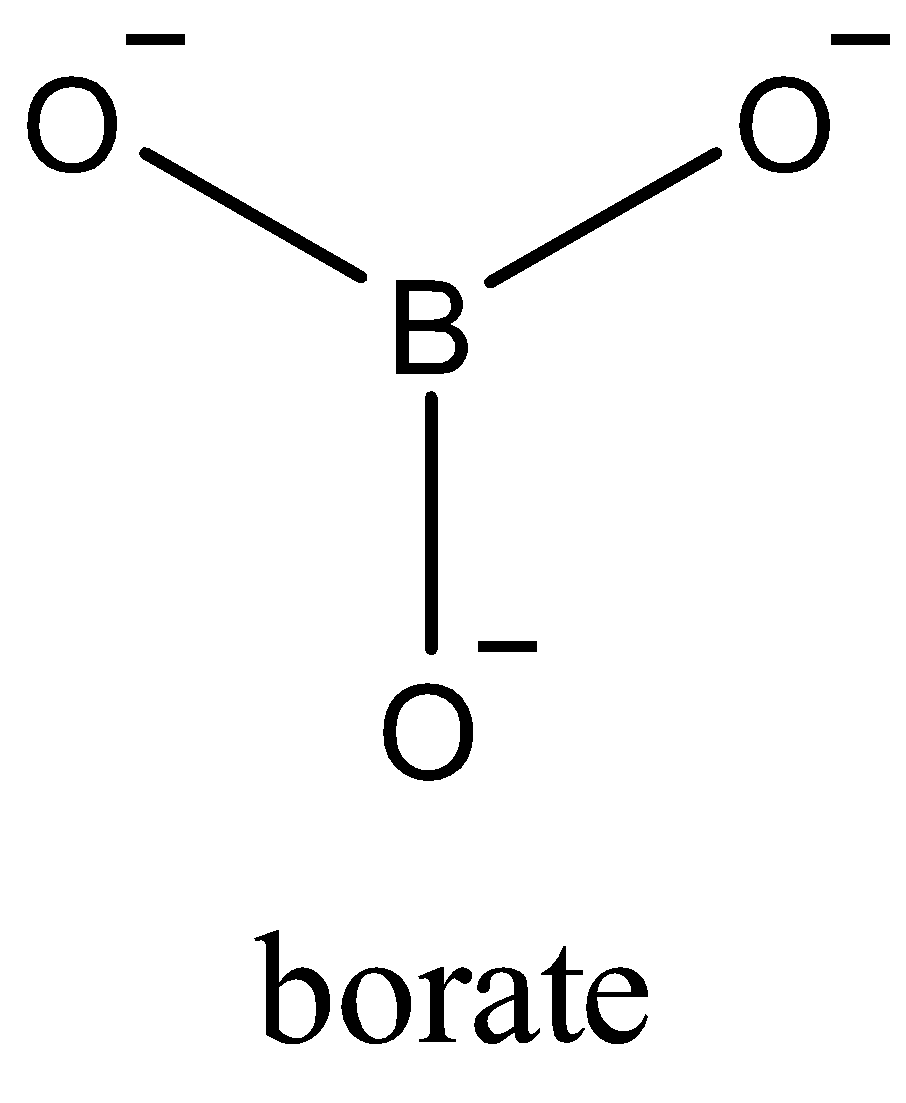
Now, since only one electron of each oxygen atom is used in the bond formation. The borate ion formed is a trivalent ion,$BO_{3}^{-3}$. Due to $s{{p}^{2}}$ the geometry of $BO_{3}^{-3}$ is trigonal planar. In the triangular $BO_{3}^{-3}$, three oxygen atoms are present at the corners of an equilateral triangle.
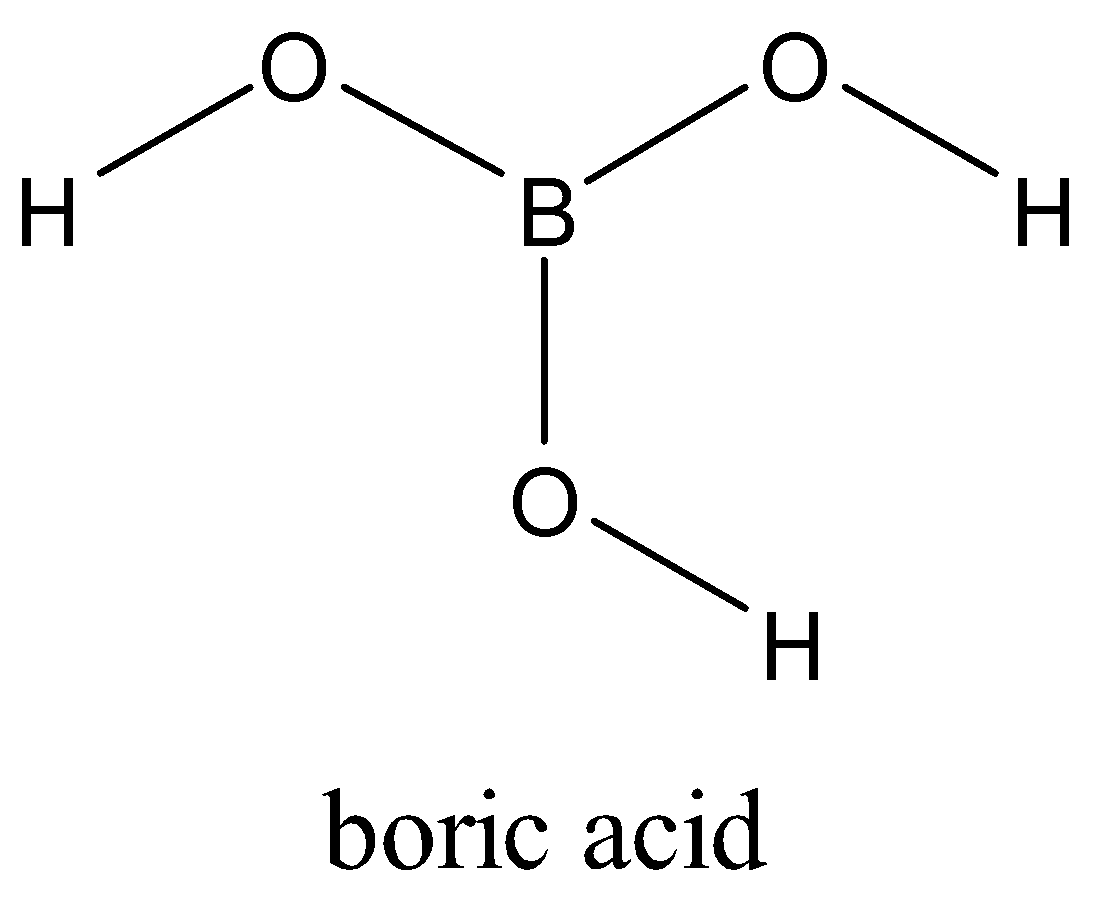
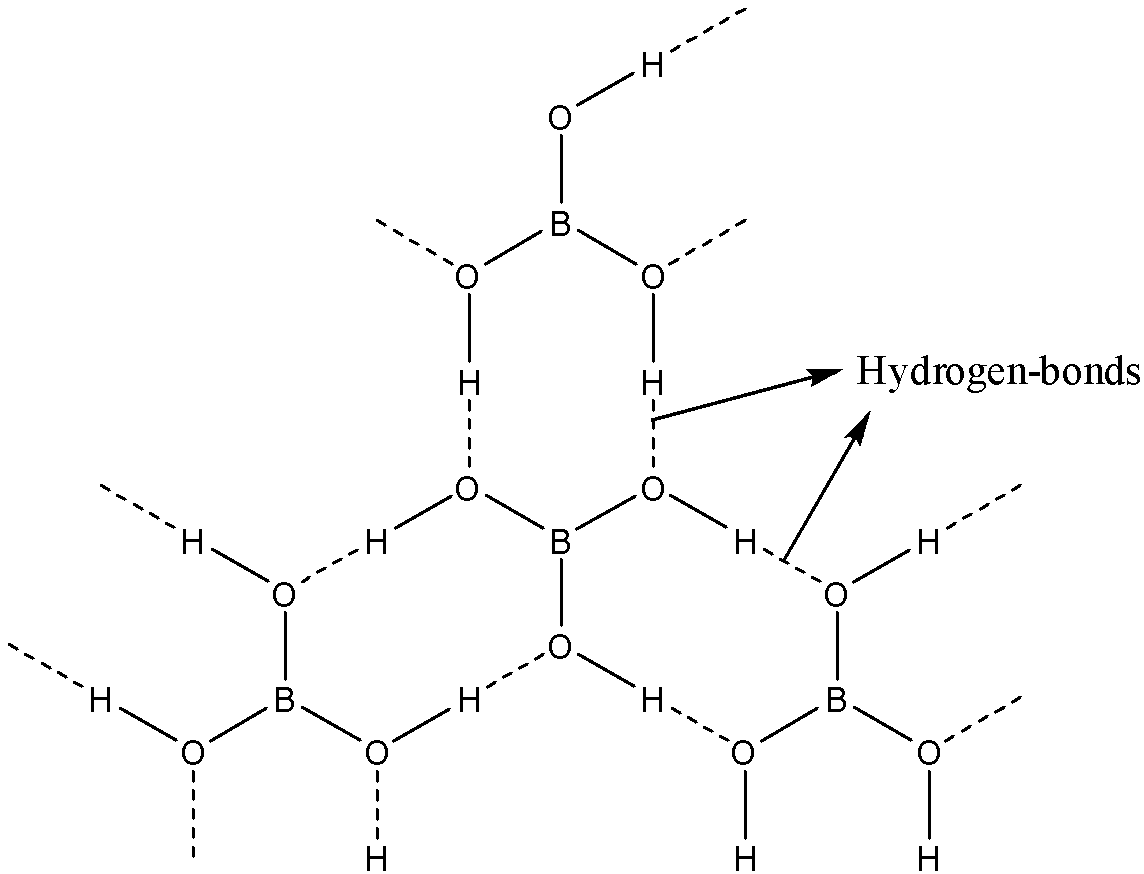
Boron is attached to three oxygen atoms and each oxygen is bonded to one hydrogen. Each such unit is ${{H}_{3}}B{{O}_{3}}$. These units are bonded together through hydrogen bonds to give a two-dimensional layered structure of boric acid.
So, the correct answer is “Option D”.
Additional Information: Boric acid or orthoboric acid is a weak monobasic acid. It does not donate protons but accepts a pair of electrons ${{H}_{3}}B{{O}_{3}}$ from and thus behaves as a Lewis acid. It has a soapy touch and is moderately soluble in water.
Note: Bonds between boron and oxygen atoms are covalent. One ${{H}_{3}}B{{O}_{3}}$ unit is joined to another through hydrogen bonding between oxygen of one ${{H}_{3}}B{{O}_{3}}$ to the hydrogen of another ${{H}_{3}}B{{O}_{3}}$.
Complete answer:
To understand the structure of boric acid (${{H}_{3}}B{{O}_{3}}$), consider the ground state electronic configuration of boron.
B (in ground state): $1{{s}^{2}}2{{s}^{2}}2{{p}^{1}}$
One electron from 2s moves to 2p-orbital in the excited state and the electronic configuration becomes: $1{{s}^{2}}2{{s}^{1}}2{{p}^{2}}$
One s and two p-orbitals are now available to bond with three oxygen atoms. Therefore, the hybridization of the central atom B is$s{{p}^{2}}$.
Now, since only one electron of each oxygen atom is used in the bond formation. The borate ion formed is a trivalent ion,$BO_{3}^{-3}$. Due to $s{{p}^{2}}$ the geometry of $BO_{3}^{-3}$ is trigonal planar. In the triangular $BO_{3}^{-3}$, three oxygen atoms are present at the corners of an equilateral triangle.

Boron is attached to three oxygen atoms and each oxygen is bonded to one hydrogen. Each such unit is ${{H}_{3}}B{{O}_{3}}$. These units are bonded together through hydrogen bonds to give a two-dimensional layered structure of boric acid.


So, the correct answer is “Option D”.
Additional Information: Boric acid or orthoboric acid is a weak monobasic acid. It does not donate protons but accepts a pair of electrons ${{H}_{3}}B{{O}_{3}}$ from and thus behaves as a Lewis acid. It has a soapy touch and is moderately soluble in water.
Note: Bonds between boron and oxygen atoms are covalent. One ${{H}_{3}}B{{O}_{3}}$ unit is joined to another through hydrogen bonding between oxygen of one ${{H}_{3}}B{{O}_{3}}$ to the hydrogen of another ${{H}_{3}}B{{O}_{3}}$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




