
Benzyne is:
A. aromatic
B. non- aromatic
C. antiaromatic
D. all of the above
Answer
576.9k+ views
Hint:For a compound to be aromatic it should be cyclic in structure, the compound should obey Huckel’s rule which means it should have (4n+2) pi number of electrons and should be flat. If the compound does not obey these rules then it is a non-aromatic compound.
Complete step by step answer:
Benzyne is the dehydrogenated form of benzene where two hydrogen atoms are removed from the benzene. Benzyne is a chemical compound having a molecular formula ${C_6}{H_4}$. The IUPAC name is cyclohexa-1,3-dien-5-yne.
Benzyne is a type of aryne where –yne stands for a triple bond. The triple bond present in the ring is non-linear because of the restriction of the six-membered ring. Benzyne is a highly reactive compound because of the strain caused by the triple bond present in the ring.
The benzyne strain energy is 50 kcal/mol greater than the strain of cyclopropane that is 28 kcal/mol and slightly has less strain than cyclopropane whose value is 54 kcal/mol.
The structure of the benzyne is shown below.
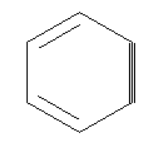
In benzyne, the triple bond is formed by the overlapping of two $s{p^2}$ orbitals which are adjacent to each other in the planar ring.
Benzyne is the aromatic compound as it is cyclic, planar in geometry. It has a conjugated 6$\pi$ electron system.
Therefore, the correct option is A.
Note:
In the structure of benzyne, the second p bond of the triple bond is present perpendicularly to the other p orbital thus it does not take part in overlapping with the aromatic $\pi$ system. Thus the two electrons related to the bond do not include in the conjugated system.
Complete step by step answer:
Benzyne is the dehydrogenated form of benzene where two hydrogen atoms are removed from the benzene. Benzyne is a chemical compound having a molecular formula ${C_6}{H_4}$. The IUPAC name is cyclohexa-1,3-dien-5-yne.
Benzyne is a type of aryne where –yne stands for a triple bond. The triple bond present in the ring is non-linear because of the restriction of the six-membered ring. Benzyne is a highly reactive compound because of the strain caused by the triple bond present in the ring.
The benzyne strain energy is 50 kcal/mol greater than the strain of cyclopropane that is 28 kcal/mol and slightly has less strain than cyclopropane whose value is 54 kcal/mol.
The structure of the benzyne is shown below.

In benzyne, the triple bond is formed by the overlapping of two $s{p^2}$ orbitals which are adjacent to each other in the planar ring.
Benzyne is the aromatic compound as it is cyclic, planar in geometry. It has a conjugated 6$\pi$ electron system.
Therefore, the correct option is A.
Note:
In the structure of benzyne, the second p bond of the triple bond is present perpendicularly to the other p orbital thus it does not take part in overlapping with the aromatic $\pi$ system. Thus the two electrons related to the bond do not include in the conjugated system.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




