
Benzyl chloride $\left( {{C}_{6}}{{H}_{5}}C{{H}_{2}}Cl \right)$ can be prepared from toluene by chlorination with?
A. $S{{O}_{2}}C{{l}_{2}}$
B. $SOC{{l}_{2}}$
C. $C{{l}_{2}}$
D. NaOCI
Answer
581.7k+ views
Hint: Benzyl chloride$\left( {{C}_{6}}{{H}_{5}}C{{H}_{2}}Cl \right)$ can be prepared from toluene by chlorination with $C{{l}_{2}}$. This is because it is found that in the presence of sunlight, $C{{l}_{2}}$ gives free radicals. This reaction is basically a free radical substitution reaction.
Complete answer:
- We can see that in toluene there is no electrophilic centre, hence oxidation takes place in the presence of sunlight using $C{{l}_{2}}$.
- $S{{O}_{2}}C{{l}_{2}}$ is found to give chlorination, when there is a nucleophilic substitution reaction on an electrophilic centre. But as we know, there is no electrophilic centre in toluene, therefore, chlorination occurs by a controlled free radical mechanism.
- NaOCI Is found to give substitution reaction, and doesn’t give free radical so it will take part in chlorination.
- We can see the chlorination reaction that is, toluene by chlorination with $C{{l}_{2}}$ will give Benzyl chloride as:
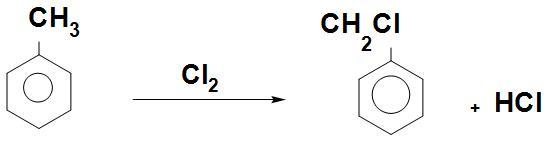
- Benzyl chloride is used in large scale in making resins, dyes, lubricants, drugs and cosmetics.
- Hence, we can conclude that the correct option is (c), that is Benzyl chloride$\left( {{C}_{6}}{{H}_{5}}C{{H}_{2}}Cl \right)$ can be prepared from toluene by chlorination with $C{{l}_{2}}$.
Note: - Industrially, Benzyl chloride can be prepared from toluene and chlorine by the gas phase photochemical mechanism. During this reaction HCl is obtained as a side product.
- It is also found that Benzyl chloride has a strong smell, inflammable to skin and can cause tearing of eyes.
Complete answer:
- We can see that in toluene there is no electrophilic centre, hence oxidation takes place in the presence of sunlight using $C{{l}_{2}}$.
- $S{{O}_{2}}C{{l}_{2}}$ is found to give chlorination, when there is a nucleophilic substitution reaction on an electrophilic centre. But as we know, there is no electrophilic centre in toluene, therefore, chlorination occurs by a controlled free radical mechanism.
- NaOCI Is found to give substitution reaction, and doesn’t give free radical so it will take part in chlorination.
- We can see the chlorination reaction that is, toluene by chlorination with $C{{l}_{2}}$ will give Benzyl chloride as:

- Benzyl chloride is used in large scale in making resins, dyes, lubricants, drugs and cosmetics.
- Hence, we can conclude that the correct option is (c), that is Benzyl chloride$\left( {{C}_{6}}{{H}_{5}}C{{H}_{2}}Cl \right)$ can be prepared from toluene by chlorination with $C{{l}_{2}}$.
Note: - Industrially, Benzyl chloride can be prepared from toluene and chlorine by the gas phase photochemical mechanism. During this reaction HCl is obtained as a side product.
- It is also found that Benzyl chloride has a strong smell, inflammable to skin and can cause tearing of eyes.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




