
Benzophenone can be obtained by ___________
A. Benzoyl chloride + Benzene + $AlC{l_3}$
B. Benzoyl chloride + Diphenyl cadmium
C. Benzoyl chloride + Phenyl magnesium chloride
D. Benzene + Carbon monoxide + $ZnC{l_2}$
Answer
566.7k+ views
Hint: We know to remember that the Friedel Crafts Acylation method is used to obtain alkanes. But in this method, the reaction proceeds in two steps. In the first step, an acylated product is formed and then on further reduction an alkane is formed.
Complete step by step answer:
We have to remember that the Friedel Crafts Acylation is the one where the reactant is an alkyl-aryl halide and benzene which reacts in the presence of a catalyst to form a benzophenone. The reaction can be represented as,
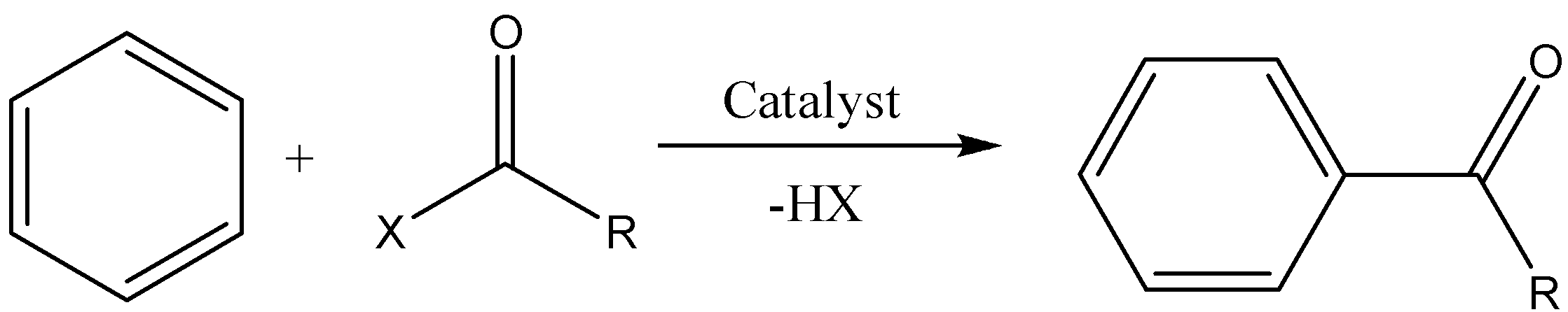
The type of reaction is an electrophilic aromatic substitution reaction. It allows the syntheses of mono-acylated products from the reaction between arenes and acyl chlorides. To process these reactions, Lewis Acid as catalyst is required as the reaction mechanism involves forming complexes of both the substrate and reactant as well.
Now let us consider the first reaction, Benzoyl chloride + Benzene + $AlC{l_3}$
We must know that the aluminium chloride is a lewis acid and majorly used in a variety of reactions especially in substitution reactions. The reaction produces benzophenone. We can write the chemical equation for this reaction as,
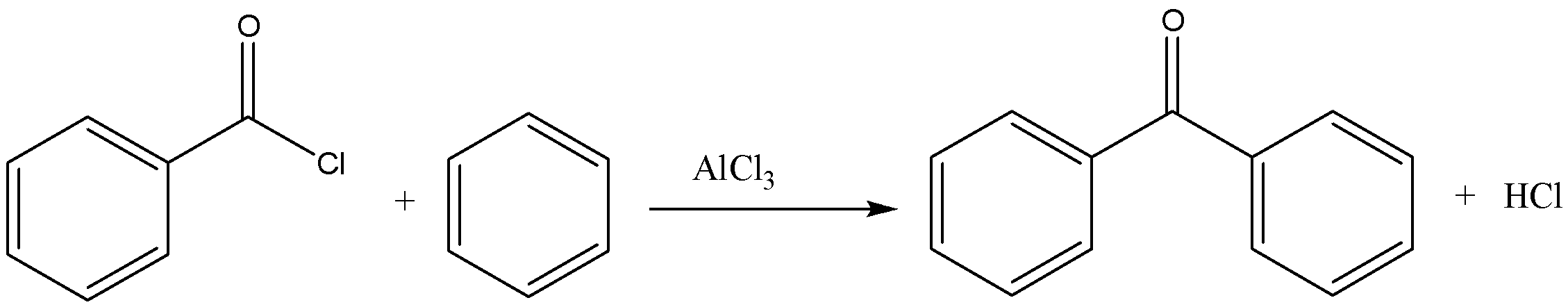
Let us look at the next reaction which is Benzoyl chloride + Diphenyl cadmium
This reaction also yields benzophenone, the reaction proceeds in the presence of dry ether and diphenyl cadmium as a catalyst. We can write the chemical equation for this reaction as,
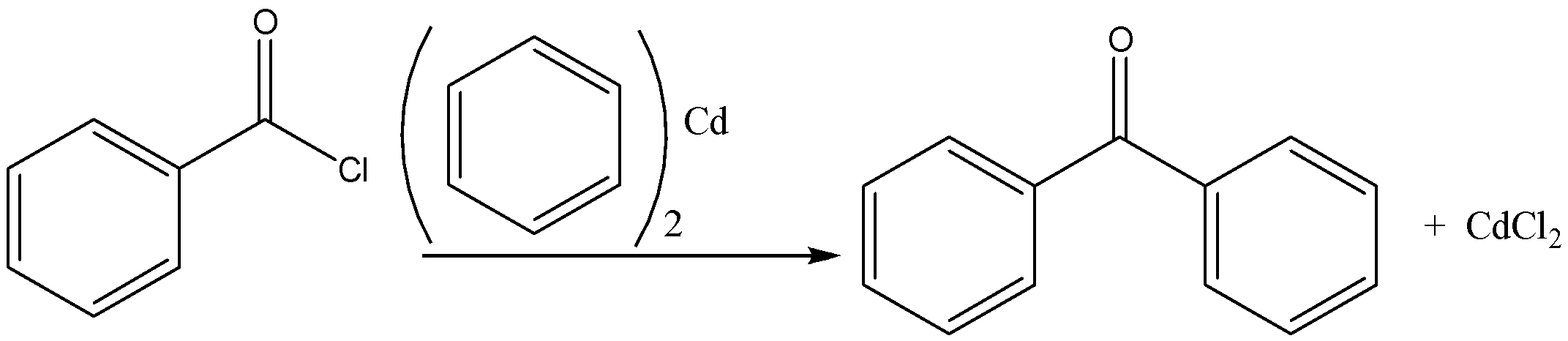
Let’s consider the third reaction, Benzoyl chloride + Phenyl magnesium chloride.
The reaction will take place and benzophenone will be formed but it will be very unstable that it will further react with the catalyst and form triphenylmethanol with phenylmagnesium bromide. Hence, this is not the correct answer.
Let us look at the last reaction, Benzene + Carbon monoxide + $ZnC{l_2}$
When this reaction takes place, the product formed is benzene diazonium chloride, which is not the correct answer.
Hence,the correct option is option A,B.
Note:
As we know that this question is an example of Friedel Crafts Acylation reaction. We have to remember that the Friedel crafts acylation is generally used to produce alkanes. But the reaction produces a stable chemical compound which is benzophenone in the first step. When the reaction proceeds, in the second step the reduction takes place where alkanes are formed using Clemmensen Reduction or Wolff-Kishner Reduction process. The Friedel Crafts Alkylation method is useful in obtaining poly alkanes, but the acylation method is useful to produce low alkanes.
Complete step by step answer:
We have to remember that the Friedel Crafts Acylation is the one where the reactant is an alkyl-aryl halide and benzene which reacts in the presence of a catalyst to form a benzophenone. The reaction can be represented as,

The type of reaction is an electrophilic aromatic substitution reaction. It allows the syntheses of mono-acylated products from the reaction between arenes and acyl chlorides. To process these reactions, Lewis Acid as catalyst is required as the reaction mechanism involves forming complexes of both the substrate and reactant as well.
Now let us consider the first reaction, Benzoyl chloride + Benzene + $AlC{l_3}$
We must know that the aluminium chloride is a lewis acid and majorly used in a variety of reactions especially in substitution reactions. The reaction produces benzophenone. We can write the chemical equation for this reaction as,

Let us look at the next reaction which is Benzoyl chloride + Diphenyl cadmium
This reaction also yields benzophenone, the reaction proceeds in the presence of dry ether and diphenyl cadmium as a catalyst. We can write the chemical equation for this reaction as,

Let’s consider the third reaction, Benzoyl chloride + Phenyl magnesium chloride.
The reaction will take place and benzophenone will be formed but it will be very unstable that it will further react with the catalyst and form triphenylmethanol with phenylmagnesium bromide. Hence, this is not the correct answer.
Let us look at the last reaction, Benzene + Carbon monoxide + $ZnC{l_2}$
When this reaction takes place, the product formed is benzene diazonium chloride, which is not the correct answer.
Hence,the correct option is option A,B.
Note:
As we know that this question is an example of Friedel Crafts Acylation reaction. We have to remember that the Friedel crafts acylation is generally used to produce alkanes. But the reaction produces a stable chemical compound which is benzophenone in the first step. When the reaction proceeds, in the second step the reduction takes place where alkanes are formed using Clemmensen Reduction or Wolff-Kishner Reduction process. The Friedel Crafts Alkylation method is useful in obtaining poly alkanes, but the acylation method is useful to produce low alkanes.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




