
Benzenediazonium chloride on treatment with water gives.
A) Benzene.
B) O-chlorophenol.
C) Anisole.
D) Phenol.
Answer
570k+ views
Hint: We know that Benzenediazonium chloride is a natural compound with the chemical formula \[\left[ {{C_6}{H_5}{N_2}} \right]Cl\]. If benzene diazonium chloride reacts with ${H_2}O$ , the diazonium group is supplanted by the \[ - OH\] group and phenol is obtained.
Complete step by step answer:
We have to remember that the benzene diazonium chloride exists as a dry strong that is dissolvable in polar solvents including water. It is the parent individual from the aryl diazonium compounds, which are generally utilized in natural science. Since the salt is flimsy, it isn't monetarily accessible however is set up upon demand.
As we know, diazonium chloride is generally an excellent leaving group (in light of the fact that it escapes as nitrogen gas) benzene diazonium Chloride gives nucleophiles replacements very easily. Water will dislodge the diazonium Chloride. Furthermore, what you'll get is phenol with additional hydrogen on \[ - {\text{ }}OH\]. Now we can write the chemical equation for this chemical reaction as,
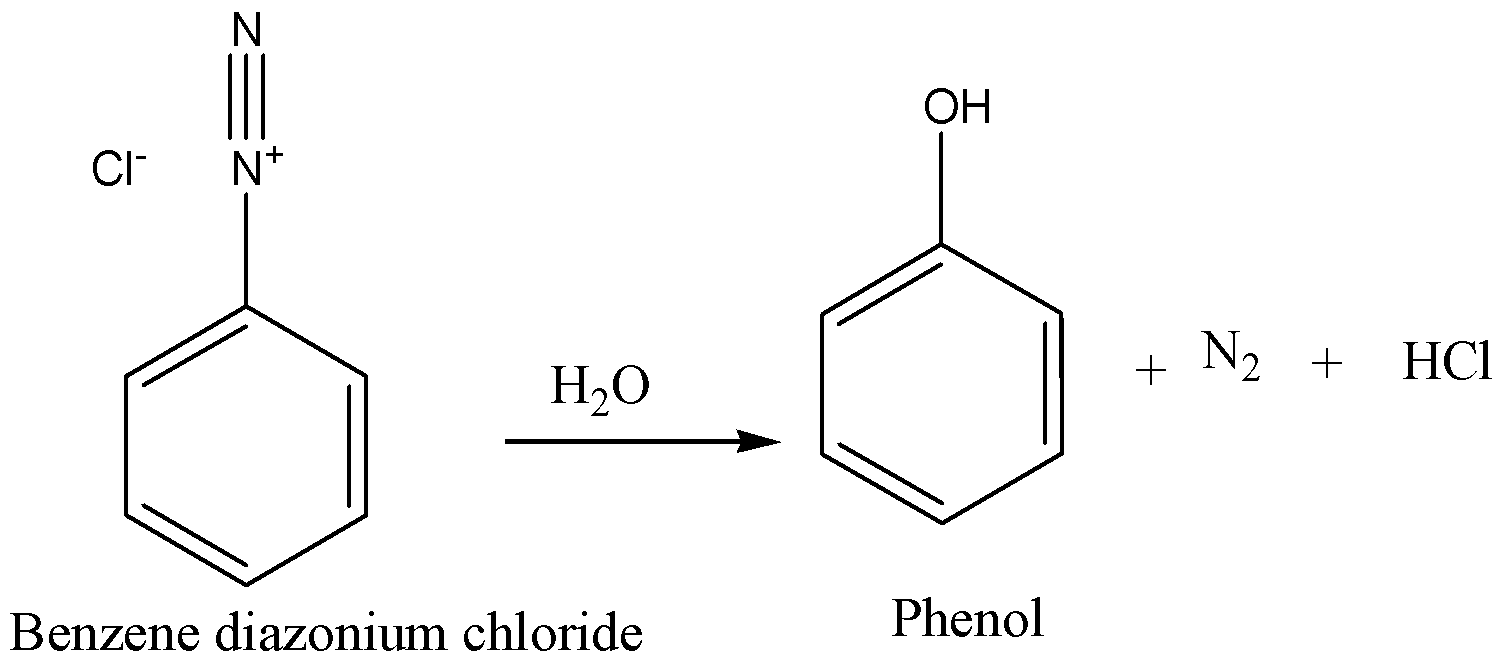
The chloride particle from the diazonium takes that additional hydrogen and hydrochloric acid. The salt of benzene diazonium is stable at \[0 - {5^ \circ }C\] .However when temperature is increased to room temperature; the nitrogen atom is replaced to give highly unstable phenyl cation. Water atoms assault the cation to give phenol.
Therefore, the option D is correct.
Note: When the diazo group can be supplanted by numerous different gatherings, normally anions, giving an assortment of subbed phenyl subsidiaries.
\[{C_6}{H_5}{N_2}^ + + N{u^ - } \to {C_6}{H_5}Nu + {N_2}\]
These changes are related with many named reactions including the Schliemann reaction, Sandmeyer reaction, and Gomberg-Bachmann reaction. A wide scope of gatherings that can be utilized to supplant Nitrogen including halide,\[S{H^ - }\], \[C{O_2}{H^ - }\],\[O{H^ - }\].Of extensive reasonable incentive in the color business are the diazo coupling reactions. 1, 3-phenyltriazine is produced when phenyl diazonium salts react with aniline.
Complete step by step answer:
We have to remember that the benzene diazonium chloride exists as a dry strong that is dissolvable in polar solvents including water. It is the parent individual from the aryl diazonium compounds, which are generally utilized in natural science. Since the salt is flimsy, it isn't monetarily accessible however is set up upon demand.
As we know, diazonium chloride is generally an excellent leaving group (in light of the fact that it escapes as nitrogen gas) benzene diazonium Chloride gives nucleophiles replacements very easily. Water will dislodge the diazonium Chloride. Furthermore, what you'll get is phenol with additional hydrogen on \[ - {\text{ }}OH\]. Now we can write the chemical equation for this chemical reaction as,

The chloride particle from the diazonium takes that additional hydrogen and hydrochloric acid. The salt of benzene diazonium is stable at \[0 - {5^ \circ }C\] .However when temperature is increased to room temperature; the nitrogen atom is replaced to give highly unstable phenyl cation. Water atoms assault the cation to give phenol.
Therefore, the option D is correct.
Note: When the diazo group can be supplanted by numerous different gatherings, normally anions, giving an assortment of subbed phenyl subsidiaries.
\[{C_6}{H_5}{N_2}^ + + N{u^ - } \to {C_6}{H_5}Nu + {N_2}\]
These changes are related with many named reactions including the Schliemann reaction, Sandmeyer reaction, and Gomberg-Bachmann reaction. A wide scope of gatherings that can be utilized to supplant Nitrogen including halide,\[S{H^ - }\], \[C{O_2}{H^ - }\],\[O{H^ - }\].Of extensive reasonable incentive in the color business are the diazo coupling reactions. 1, 3-phenyltriazine is produced when phenyl diazonium salts react with aniline.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




