
How would you balance the following equation? $Zn\, + \,S\, \to \,ZnS$
Answer
552.3k+ views
Hint:Make the half-cell reaction balance and then add them so that there are equal electrons on both sides and they will get cancelled. This method can be used to check the number of electrons while applying the Nernst equation for finding the potential difference. See which of the reactants is getting oxidized and which is getting reduced.
Complete step-by-step answer:Here, the above reaction given is the redox reaction, it means one species is getting reduced and another is getting oxidized. In the reduction reaction, there is a change in oxidation state from higher to lower, while in case of oxidation reaction there is change from lower oxidation state to higher oxidation state. If we see this reaction we have it as,
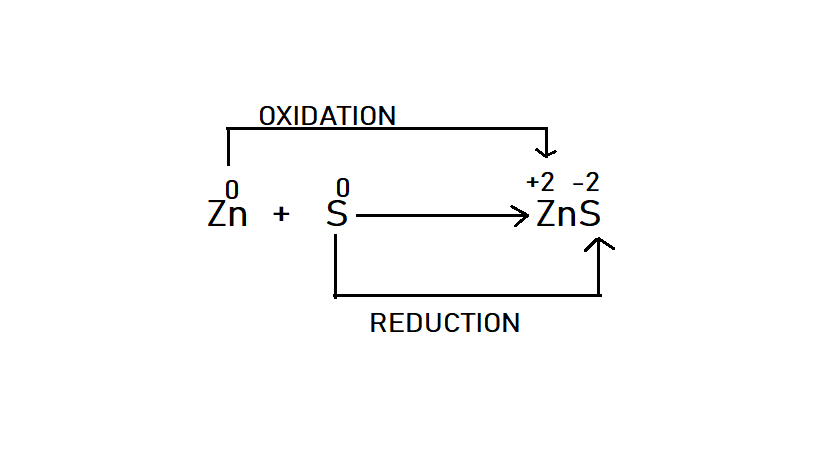
Here, as we see that zinc is changing from oxidation state $(0)\,to\,( + 2)$ while sulphur is getting reduced it means its oxidation state is changing from $(0)\,to\,( - 2)$. So, we can write the half-cell which basically reacts by having electrons transfer from one species to another. Let’s start with oxidation half-cell reaction,
$Zn\,\xrightarrow{{Oxidation}}\,Z{n^{ + 2}}\, + \,2{e^ - }$
Zinc is getting oxidized to zinc ion, similarly we are having sulphur which is getting reduced so reduction half-cell reaction is,
$S\, + 2{e^ - }\,\xrightarrow{{\operatorname{Re} duction}}\,{S^{ - 2}}$
Now on addition we get the balanced equation as, $Zn\, + \,S\, \to \,ZnS$
It means atoms in the reactions are equal and similarly the number of electrons are also equal. Therefore we can say the above reaction is balanced.
Note:The redox reactions are mainly balanced using two methods one is oxidation reduction method and the other one is electron method. So both methods at the end make the number of atoms equal. Sometimes cases arise of hydrates where water of crystallization is also involved, thus in those waters we just have to remove the water of crystallization and then balance the equation as discussed above.
Complete step-by-step answer:Here, the above reaction given is the redox reaction, it means one species is getting reduced and another is getting oxidized. In the reduction reaction, there is a change in oxidation state from higher to lower, while in case of oxidation reaction there is change from lower oxidation state to higher oxidation state. If we see this reaction we have it as,

Here, as we see that zinc is changing from oxidation state $(0)\,to\,( + 2)$ while sulphur is getting reduced it means its oxidation state is changing from $(0)\,to\,( - 2)$. So, we can write the half-cell which basically reacts by having electrons transfer from one species to another. Let’s start with oxidation half-cell reaction,
$Zn\,\xrightarrow{{Oxidation}}\,Z{n^{ + 2}}\, + \,2{e^ - }$
Zinc is getting oxidized to zinc ion, similarly we are having sulphur which is getting reduced so reduction half-cell reaction is,
$S\, + 2{e^ - }\,\xrightarrow{{\operatorname{Re} duction}}\,{S^{ - 2}}$
Now on addition we get the balanced equation as, $Zn\, + \,S\, \to \,ZnS$
It means atoms in the reactions are equal and similarly the number of electrons are also equal. Therefore we can say the above reaction is balanced.
Note:The redox reactions are mainly balanced using two methods one is oxidation reduction method and the other one is electron method. So both methods at the end make the number of atoms equal. Sometimes cases arise of hydrates where water of crystallization is also involved, thus in those waters we just have to remove the water of crystallization and then balance the equation as discussed above.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




