
Assertion-The central carbon-carbon bond in butane is larger than that in 1,3-butadiene
Reason- The more is s-character in hybridization, the bond length is less
(A)Assertion is true, Reason is true and reason is correct explanation for Assertion
(B)Assertion is true, reason is true and Reason is not the correct explanation for Assertion.
(C)Assertion is true, Reason is false
(D)Assertion is false, Reason is true
Answer
592.8k+ views
Hint: Bond order is directly proportional to percentage s-character. The greater the s-character, the stronger the bond is. Conjugation in double bonds, imparts partial double bond character to single and double bonds.
Complete answer:
Butane is a hydrocarbon of alkane class with carbon atoms of hybridization of $s{{p}^{3}}$with normal C-C bond lengths. They have 25% s-character. The structure of butane molecule is given as follows:
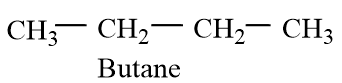
And Butadiene is an alkene compound with 2 double bonds and the two double bonds are conjugated. The double bonds show conjugation, which increases the stability of the diene molecule. The molecule forms two resonating molecules. The carbon atoms have $s{{p}^{2}}$.
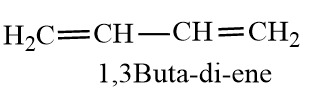
The double is conjugated between carbon-1 to carbon-3. The carbon-carbon bond in the centre of butadiene has a partial double bond character, due to which it is less than normal C-C bond length.The length of C-C bond is between single bond of C-C and double bond of C-C. The double bonds have 33% s-character, increased s-character increases, Bond order and decreases bond length. So percentage s-character is inversely proportional to bond length.
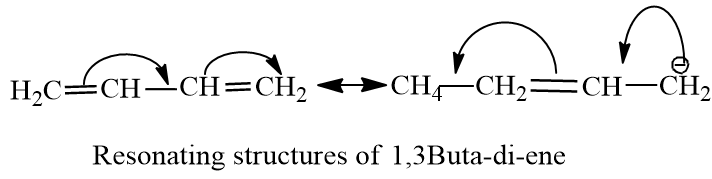
The percentage s-character of butane is less than butadiene , therefore , the bond length of the central carbon-carbon bond is more than that of butadiene.
Therefore, the Assertion is true, and the reason is the correct explanation of Assertion.
So, the correct answer is “Option A”.
Note: As s-character increases, electronegativity of carbon atom increases, bond angle, bond order increases and bond strength also increases. Since bond strength is increasing, bond enthalpy also increases.
Complete answer:
Butane is a hydrocarbon of alkane class with carbon atoms of hybridization of $s{{p}^{3}}$with normal C-C bond lengths. They have 25% s-character. The structure of butane molecule is given as follows:
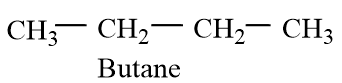
And Butadiene is an alkene compound with 2 double bonds and the two double bonds are conjugated. The double bonds show conjugation, which increases the stability of the diene molecule. The molecule forms two resonating molecules. The carbon atoms have $s{{p}^{2}}$.

The double is conjugated between carbon-1 to carbon-3. The carbon-carbon bond in the centre of butadiene has a partial double bond character, due to which it is less than normal C-C bond length.The length of C-C bond is between single bond of C-C and double bond of C-C. The double bonds have 33% s-character, increased s-character increases, Bond order and decreases bond length. So percentage s-character is inversely proportional to bond length.

The percentage s-character of butane is less than butadiene , therefore , the bond length of the central carbon-carbon bond is more than that of butadiene.
Therefore, the Assertion is true, and the reason is the correct explanation of Assertion.
So, the correct answer is “Option A”.
Note: As s-character increases, electronegativity of carbon atom increases, bond angle, bond order increases and bond strength also increases. Since bond strength is increasing, bond enthalpy also increases.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




