
Assertion-Intake of small quantities of methanol can be lethal.
Reason- Methanol $\left( {{\text{C}}{{\text{H}}_3}{\text{OH}}} \right)$ is highly toxic and unfit for consumption. Methanol ingested in large quantities is metabolized to formic acid or formate salts, which is poisonous to the central nervous system and may cause blindness, coma and death.
A) Both Assertion and Reason are correct and Reason is the correct explanation for Assertion.
B) Both Assertion and Reason are correct but Reason is not the correct explanation for Assertion.
C) Assertion is correct but Reason is incorrect.
D) Both Assertion and Reason are correct.
Answer
591.9k+ views
Hint: Methanol is very poisonous. If it is inhaled or ingested even in small quantities, it may lead to death as it forms formic acid or formate when acted upon by biological enzymes.
Complete step by step answer:
Methanol is poisonous. Methanol if taken in large quantities gets converted to methanal (formaldehyde) in the liver due to oxidation. Methanal is toxic and carcinogenic. Methanal rapidly reacts with biological enzymes and gets metabolized to formic acid or formate salts. This substance gets coagulated in the cells the same way eggs get coagulated on boiling and this blocks the optic nerve which causes blindness. It is so harmful it causes skin damage if a person comes in direct contact with it. If taken in large quantities it can cause coma or death.
So assertion and reason both are true and reason is the correct explanation of assertion.
Hence, the correct option is “A”.
Additional Information:
Methanol is the first member of the alcohol family which is a clear, colorless liquid. Its chemical formula is $\left( {{\text{C}}{{\text{H}}_3}{\text{OH}}} \right)$ and structure is-
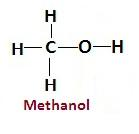
It is also called methyl alcohol because it contains one methyl group and functional group of alcohol. Due to the presence of hydroxyl groups it can form hydrogen bonds easily with other hydrogen bond acceptors like water. It is highly inflammable liquid and highly polar in nature which makes it a good solvent.
Note:
Methanol is the simplest member of alcohol family which is widely used for industrial purposes .It is used in-
-It is used in production of many chemical compounds like acetic acid and formaldehyde, olefins, aromatic compounds and methyl-esters.
-It is used as antifreeze in many pipelines as it lowers the freezing point of liquid.
-It serves as a carbon based food source for denitrifying bacteria so it is used in sewage treatment.
-It is used as a fuel in internal combustion engines but it corrodes aluminium and other metals.
Complete step by step answer:
Methanol is poisonous. Methanol if taken in large quantities gets converted to methanal (formaldehyde) in the liver due to oxidation. Methanal is toxic and carcinogenic. Methanal rapidly reacts with biological enzymes and gets metabolized to formic acid or formate salts. This substance gets coagulated in the cells the same way eggs get coagulated on boiling and this blocks the optic nerve which causes blindness. It is so harmful it causes skin damage if a person comes in direct contact with it. If taken in large quantities it can cause coma or death.
So assertion and reason both are true and reason is the correct explanation of assertion.
Hence, the correct option is “A”.
Additional Information:
Methanol is the first member of the alcohol family which is a clear, colorless liquid. Its chemical formula is $\left( {{\text{C}}{{\text{H}}_3}{\text{OH}}} \right)$ and structure is-

It is also called methyl alcohol because it contains one methyl group and functional group of alcohol. Due to the presence of hydroxyl groups it can form hydrogen bonds easily with other hydrogen bond acceptors like water. It is highly inflammable liquid and highly polar in nature which makes it a good solvent.
Note:
Methanol is the simplest member of alcohol family which is widely used for industrial purposes .It is used in-
-It is used in production of many chemical compounds like acetic acid and formaldehyde, olefins, aromatic compounds and methyl-esters.
-It is used as antifreeze in many pipelines as it lowers the freezing point of liquid.
-It serves as a carbon based food source for denitrifying bacteria so it is used in sewage treatment.
-It is used as a fuel in internal combustion engines but it corrodes aluminium and other metals.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




