
ASSERTION
STATEMENT-1: m $ - $ Chlorobenzoicacid is a stronger acid than p $ - $ chlorobenzoic acid.
REASON
STATEMENT-2: In m $ - $ chlorobenzoicacid, both $ - $ I effect and $ + $ M effect of Cl operate, but in p $ - $ isomer only $ + $ M effect of Cl operates.
A. STATEMENT-1 is True, STATEMENT-2 is True; STATEMENT-2 is a correct explanation for STATEMENT-1
B. STATEMENT-1 is True, STATEMENT-2 is True; STATEMENT-2 is NOT a correct explanation for STATEMENT-1
C. STATEMENT-1 is True, STATEMENT-2 is False
D. STATEMENT-1 is False, STATEMENT-2 is True
Answer
573.3k+ views
Hint: The mesomeric effect is the electron-withdrawing or the electron releasing effect of substituent groups on a compound. The effect can be positive ($ + $M).
The electron-withdrawing nature of groups of atoms is called a negative inductive effect.
Hence, both mesomeric and inductive effects have an electron-withdrawing nature.
To check the acidity of an organic compound, one can remove the proton and then check the stability of the resulting conjugate base so formed. More is the stability of the conjugate base, stronger is the acid.
Complete step by step answer:
(Negative Inductive Effect)When an electronegative atom, for example, halogens, is introduced to a chain of atoms (generally carbon atoms), the resulting unequal sharing of electrons generates a positive charge which is transmitted through the chain.
This causes a permanent dipole to be created in the molecule where the electronegative atom holds a negative charge and the corresponding effect is referred to as the electron-withdrawing inductive effect.
(Positive mesomeric effect)When the electrons are transferred from a particular group towards a conjugate system, thus increasing the electron density of the conjugated system then such a phenomenon is referred to as (+M) effect or positive mesomeric effect.
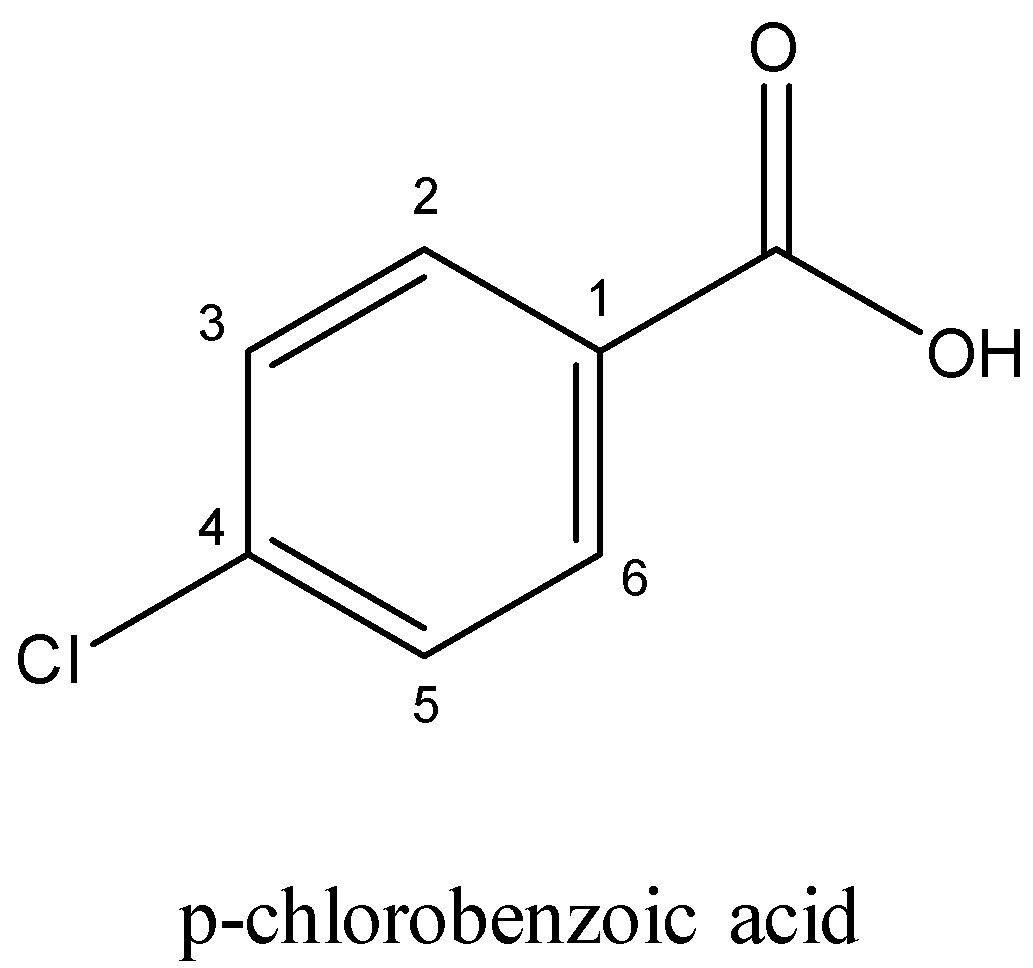
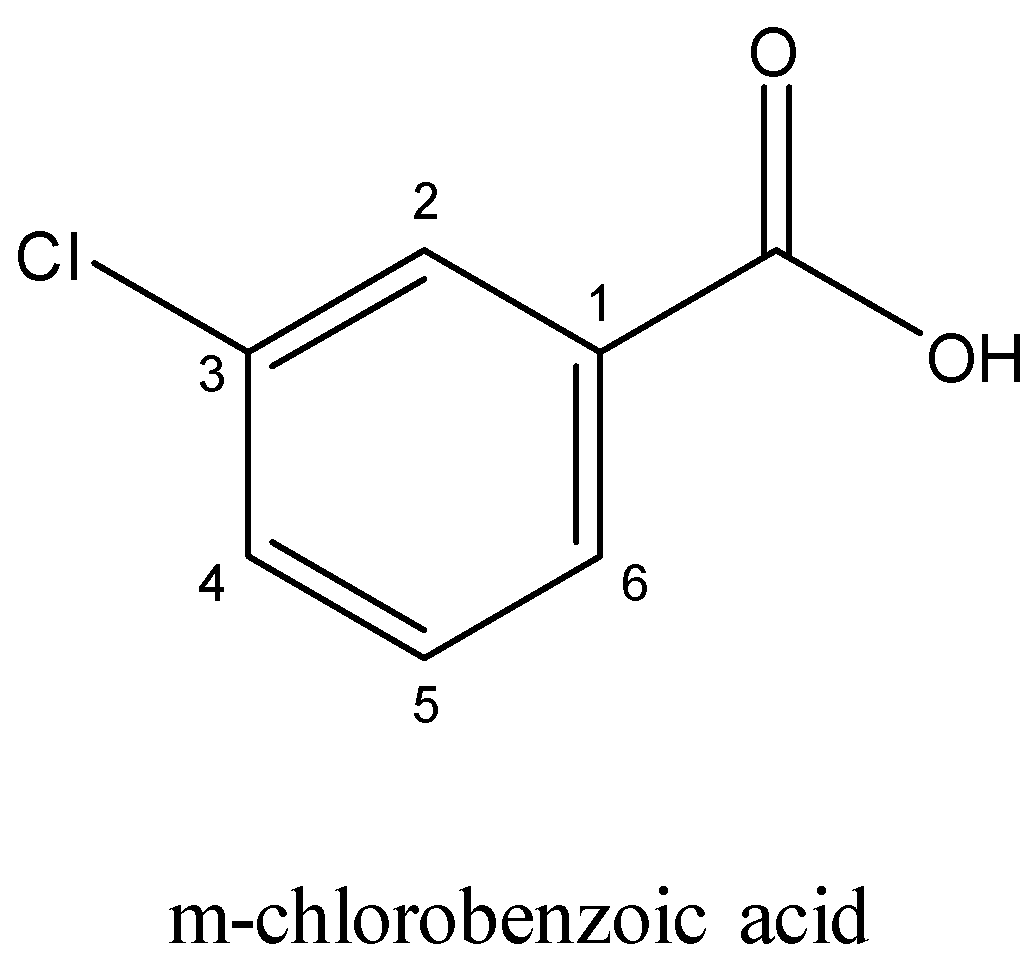
In case of m $ - $ Chlorobenzoicacid and p $ - $ chlorobenzoicacid,
In m $ - $ chlorobenzoicacid, we can say that only $ - $ I (negative inductive effect) effect operates, but in p-isomer, both $ - $ I as well as $ + $ M (positive mesomeric effect) effect of CI operates and $ + $ M effect reduces the strength of the acid. Both the electron-withdrawing groups in the latter have a greater impact in reducing the acidity of the compound. Also, Chlorine is more deactivating
So, the correct answer is Option C.
Note: 1. With the $ + $ M effect, the group will have either a lone pair of electrons or should have a negative charge.
2. The $ + $ M effect would give a negative charge to the conjugate system or it can be said that the electron density increases on the conjugate system due to this. These conjugate systems show more reactivity towards electrophiles and less reactivity towards nucleophiles.
3. When a group displaying the $ - $ I effect is bonded to a molecule, the electron density of the resulting molecule effectively reduces, thus making it more likely to accept electrons and thereby increasing the acidity of the molecule.
4. When a $ + $ I group attaches itself to a molecule, then there is an increase in the electron density of the molecule. This increases the basicity of the molecule because it is now more capable of donating electrons.
The electron-withdrawing nature of groups of atoms is called a negative inductive effect.
Hence, both mesomeric and inductive effects have an electron-withdrawing nature.
To check the acidity of an organic compound, one can remove the proton and then check the stability of the resulting conjugate base so formed. More is the stability of the conjugate base, stronger is the acid.
Complete step by step answer:
(Negative Inductive Effect)When an electronegative atom, for example, halogens, is introduced to a chain of atoms (generally carbon atoms), the resulting unequal sharing of electrons generates a positive charge which is transmitted through the chain.
This causes a permanent dipole to be created in the molecule where the electronegative atom holds a negative charge and the corresponding effect is referred to as the electron-withdrawing inductive effect.
(Positive mesomeric effect)When the electrons are transferred from a particular group towards a conjugate system, thus increasing the electron density of the conjugated system then such a phenomenon is referred to as (+M) effect or positive mesomeric effect.
In case of m $ - $ Chlorobenzoicacid and p $ - $ chlorobenzoicacid,
In m $ - $ chlorobenzoicacid, we can say that only $ - $ I (negative inductive effect) effect operates, but in p-isomer, both $ - $ I as well as $ + $ M (positive mesomeric effect) effect of CI operates and $ + $ M effect reduces the strength of the acid. Both the electron-withdrawing groups in the latter have a greater impact in reducing the acidity of the compound. Also, Chlorine is more deactivating


So, the correct answer is Option C.
Note: 1. With the $ + $ M effect, the group will have either a lone pair of electrons or should have a negative charge.
2. The $ + $ M effect would give a negative charge to the conjugate system or it can be said that the electron density increases on the conjugate system due to this. These conjugate systems show more reactivity towards electrophiles and less reactivity towards nucleophiles.
3. When a group displaying the $ - $ I effect is bonded to a molecule, the electron density of the resulting molecule effectively reduces, thus making it more likely to accept electrons and thereby increasing the acidity of the molecule.
4. When a $ + $ I group attaches itself to a molecule, then there is an increase in the electron density of the molecule. This increases the basicity of the molecule because it is now more capable of donating electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




