
Assertion:
In $S{O_2}$, the bond angle is \[119^\circ \] whereas in $S{O_3}$, the bond angle is \[{120^ \circ }\].
Reason:
S atom in both $S{O_2}$ and $S{O_3}$ is \[s{p^2}\]-hybridized.
A. If both assertion and reason are CORRECT and reason is the CORRECT explanation of the assertion.
B. If both assertion and reason are CORRECT, but reason is NOT THE CORRECT explanation of the assertion.
C. If assertion is CORRECT, but reason is INCORRECT.
D. If assertion is INCORRECT, but reason is CORRECT.
Answer
581.7k+ views
Hint: It is well known to us that the shape or geometry of the molecule depends on the number of lone pair and bonded pair of electrons around the central atom. If a lone pair of electrons are present then the shape of the molecule is distorted and if only a bonded pair of electrons are present the shape of the molecule is having regular geometry. Mixing atomic orbitals of the same atom to form equal number, sized, energized orbital is hybridization.
Complete step by step answer:
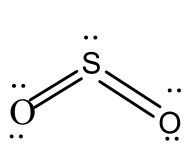
In $S{O_3}$ the central atom S must be excited to provide six unpaired electrons to form six bonds. The three $\pi $bonds are ignored in determining the shape of the molecule. The three sigma orbitals are directed towards the corners of an equilateral triangle, and the molecule becomes a completely regular plane triangle. The $\pi $ bond shortens the bond lengths, but does not affect the shape. The $\pi $ bonds formed all are of equal strength and it is delocalized on several atoms.
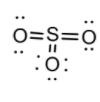
In $S{O_2}$, S shows oxidation states(+II), (+IV), (+VI) while O has two valencies. We know that triatomic molecules are either bent or linear. The S atom must be excited to provide four unpaired electrons. The two unpaired electron pairs which form the $\pi $ bonds do not affect the shape of the molecule. The remaining three orbitals point to the corners of the triangle, and result in planar triangular structure for the molecule with two corners occupied by O atoms and one corner occupied by a lone pair. The $S{O_2}$is thus v-shaped or angular. As we discussed above the $\pi $bonds do not alter the shape but merely shorten the bond lengths. The bond angle is reduced from the ideal value of \[{120^ \circ }\] to \[119^\circ \]because of the repulsion of the lone pair of atoms.
The $S{O_2}$ molecule has a dipole moment with a bond angle of 119°, in $S{O_2}$, we have 2 double bonds and one lone pair of electrons. There are three electron regions so the bond angle is 120°. The extra repulsion of the lone pair and double bonds accounts for the reduction to 119°.
Sulfur atom in both $S{O_2}$ and $S{O_3}$ is \[s{p^2}\] hybridized. But it is related to dipole moment and assertion is the example for bent angle so here reason is unable to give an explanation of assertion.
Therefore, the correct option is Option B.
Note:
The certain facts that we should know are Lone pairs of electrons occupy more space than bonding electrons. The presence of lone pair electrons will distort predicted bond angles. Dipole moment is the product of charges and the distance between the two ends.
Complete step by step answer:
In $S{O_3}$ the central atom S must be excited to provide six unpaired electrons to form six bonds. The three $\pi $bonds are ignored in determining the shape of the molecule. The three sigma orbitals are directed towards the corners of an equilateral triangle, and the molecule becomes a completely regular plane triangle. The $\pi $ bond shortens the bond lengths, but does not affect the shape. The $\pi $ bonds formed all are of equal strength and it is delocalized on several atoms.
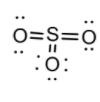
In $S{O_2}$, S shows oxidation states(+II), (+IV), (+VI) while O has two valencies. We know that triatomic molecules are either bent or linear. The S atom must be excited to provide four unpaired electrons. The two unpaired electron pairs which form the $\pi $ bonds do not affect the shape of the molecule. The remaining three orbitals point to the corners of the triangle, and result in planar triangular structure for the molecule with two corners occupied by O atoms and one corner occupied by a lone pair. The $S{O_2}$is thus v-shaped or angular. As we discussed above the $\pi $bonds do not alter the shape but merely shorten the bond lengths. The bond angle is reduced from the ideal value of \[{120^ \circ }\] to \[119^\circ \]because of the repulsion of the lone pair of atoms.

The $S{O_2}$ molecule has a dipole moment with a bond angle of 119°, in $S{O_2}$, we have 2 double bonds and one lone pair of electrons. There are three electron regions so the bond angle is 120°. The extra repulsion of the lone pair and double bonds accounts for the reduction to 119°.
Sulfur atom in both $S{O_2}$ and $S{O_3}$ is \[s{p^2}\] hybridized. But it is related to dipole moment and assertion is the example for bent angle so here reason is unable to give an explanation of assertion.
Therefore, the correct option is Option B.
Note:
The certain facts that we should know are Lone pairs of electrons occupy more space than bonding electrons. The presence of lone pair electrons will distort predicted bond angles. Dipole moment is the product of charges and the distance between the two ends.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




