
Assertion: For liquid dishwashing, non-ionic type of detergent used.
Reason: Remove grease and oil by micelle formation.
A.Both the assertion and reason are correct and reason is the correct explanation for assertion.
B.Both the assertion and reason are correct and reason is not the correct explanation for assertion.
C.Assertion is incorrect but reason is incorrect.
D.Both Assertion and reason are incorrect.
Answer
565.2k+ views
Hint:We need to know that the amphipathic particles that contain charged hydrophilic or polar gatherings toward the finish of long lipophilic hydrocarbon bunch are called cleansers. The charged hydrophilic gathering is additionally called the head and the long lipophilic hydrocarbon bunch is known as the tail. Cleansers are otherwise called surfactants as they can diminish the surface pressure of water. A micelle is a total of surfactant particles scattered in a fluid, shaping a colloidal suspension.
Complete step by step answer:
We have to remember that the nonionic cleansers are utilized in dish washing fluids. Since the cleanser doesn't have any ionic gatherings, it doesn't respond with hard water particles yet can eliminate oil a lot by micelle formation. Hence, both Assertion and Reason are right and Reason is the right clarification for Assertion. Hence option A is correct.
Structure of soap:
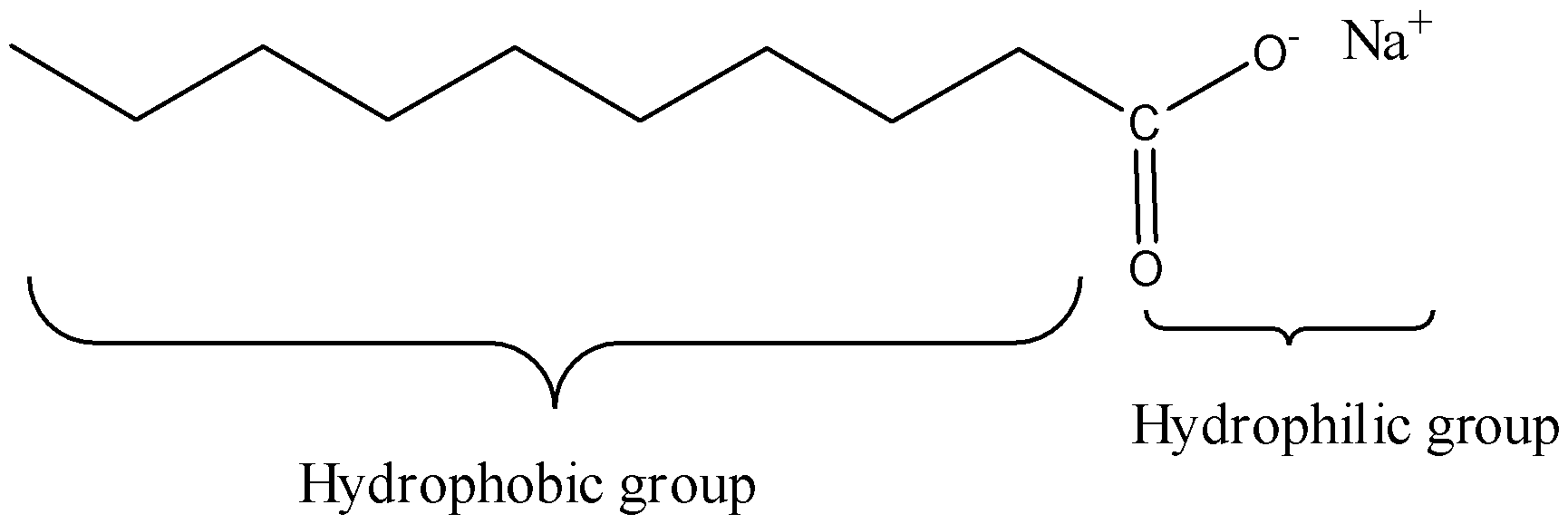
We must know that the soaps are sodium or potassium unsaturated fats salts, delivered from the hydrolysis of fats in a substance response called saponification. Each cleanser particle has a long hydrocarbon chain, now and again called its 'tail', with a carboxylate 'head'. In water, the sodium or potassium particles coast free, leaving a contrarily charged head. The natural piece of characteristic cleanser is an adversely charged, polar atom. Its hydrophilic (water-adoring) carboxylate group communicates with water particles by means of particle dipole collaborations and hydrogen holding. The hydrophobic (water-dreading) part of a cleanser atom, its long, nonpolar hydrocarbon chain, doesn't cooperate with water particles. The hydrocarbon suffixes are pulled in to one another by scattering powers and bunching together, shaping structures called micelles. In these micelles, the carboxylate bunches structure a contrarily charged round surface, with the hydrocarbon chains inside the circle. Since they are adversely charged, cleanser micelles repulse one another and stay scattered in water.
Note:
We realize that washing cleansers are the potassium salts of long-chain unsaturated fats though washing cleansers which are additionally called cleansers are sodium salts of long-chain unsaturated fats. Washing cleansers are potassium salts of long-chain unsaturated fat and the anionic gathering present in cleanser is Detergents are sodium salts are long-chain unsaturated fats and the anionic gathering present depends on the idea of the antacid utilized in cleanser creation, cleansers have various properties. Sodium hydroxide gives the hard cleanser which is utilized for washing while potassium hydroxide gives a delicate cleanser that is utilized for washing.
The principle contrast among cleanser and cleansers are:
-Soaps are set up from vegetable oil and creature fats while cleansers are set up from coal and oil.
-Soaps are biodegradable while cleansers are non-biodegradable.
-Soaps don't effortlessly shape foam in hard water however cleansers effectively structure foam in hard water. Soaps show feeble purifying activity so they can't be utilized in acidic water then again cleansers show solid purging activity so they can be utilized in acidic water.
Complete step by step answer:
We have to remember that the nonionic cleansers are utilized in dish washing fluids. Since the cleanser doesn't have any ionic gatherings, it doesn't respond with hard water particles yet can eliminate oil a lot by micelle formation. Hence, both Assertion and Reason are right and Reason is the right clarification for Assertion. Hence option A is correct.
Structure of soap:
We must know that the soaps are sodium or potassium unsaturated fats salts, delivered from the hydrolysis of fats in a substance response called saponification. Each cleanser particle has a long hydrocarbon chain, now and again called its 'tail', with a carboxylate 'head'. In water, the sodium or potassium particles coast free, leaving a contrarily charged head. The natural piece of characteristic cleanser is an adversely charged, polar atom. Its hydrophilic (water-adoring) carboxylate group communicates with water particles by means of particle dipole collaborations and hydrogen holding. The hydrophobic (water-dreading) part of a cleanser atom, its long, nonpolar hydrocarbon chain, doesn't cooperate with water particles. The hydrocarbon suffixes are pulled in to one another by scattering powers and bunching together, shaping structures called micelles. In these micelles, the carboxylate bunches structure a contrarily charged round surface, with the hydrocarbon chains inside the circle. Since they are adversely charged, cleanser micelles repulse one another and stay scattered in water.

Note:
We realize that washing cleansers are the potassium salts of long-chain unsaturated fats though washing cleansers which are additionally called cleansers are sodium salts of long-chain unsaturated fats. Washing cleansers are potassium salts of long-chain unsaturated fat and the anionic gathering present in cleanser is Detergents are sodium salts are long-chain unsaturated fats and the anionic gathering present depends on the idea of the antacid utilized in cleanser creation, cleansers have various properties. Sodium hydroxide gives the hard cleanser which is utilized for washing while potassium hydroxide gives a delicate cleanser that is utilized for washing.
The principle contrast among cleanser and cleansers are:
-Soaps are set up from vegetable oil and creature fats while cleansers are set up from coal and oil.
-Soaps are biodegradable while cleansers are non-biodegradable.
-Soaps don't effortlessly shape foam in hard water however cleansers effectively structure foam in hard water. Soaps show feeble purifying activity so they can't be utilized in acidic water then again cleansers show solid purging activity so they can be utilized in acidic water.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




