
Arrange the following in increasing order of expected enol content:
i.) $C{{H}_{3}}COC{{H}_{2}}CHO$
ii.) $C{{H}_{3}}COC{{H}_{3}}$
iii.) $C{{H}_{3}}CHO$
iv.) $C{{H}_{3}}COC{{H}_{2}}COC{{H}_{3}}$
a.) iii < i < ii < iv
b.) iii < ii < i < iv
c.) i < iv < ii < iii
d.) iv < i < ii < iii
Answer
570k+ views
Hint: In two tautomeric form of a compound hydrogen bonding, resonance, hyperconjugation etc. have the tendency to stabilize the enolic form that’s why presence of all or one of these factors increases the enol content of a compound or makes enol form more stable.
Complete Solution :
- Among all 4 compounds $C{{H}_{3}}COC{{H}_{2}}COC{{H}_{3}}$ acetyl acetone has highest enol content.
- Here you can observe in the enol form of acetylacetone that hydrogen of OH group is forming a hydrogen bond with oxygen of carbonyl carbon, along with that alpha carbon from double bond has three alpha hydrogens, so this double bond will be stabilized by hyperconjugation.
- So hydrogen bonding and hyperconjugation together are increasing the stability of enol form of acetylacetone and that’s why it has the highest enolic content.
Enolic form of acetylacetone is shown below:
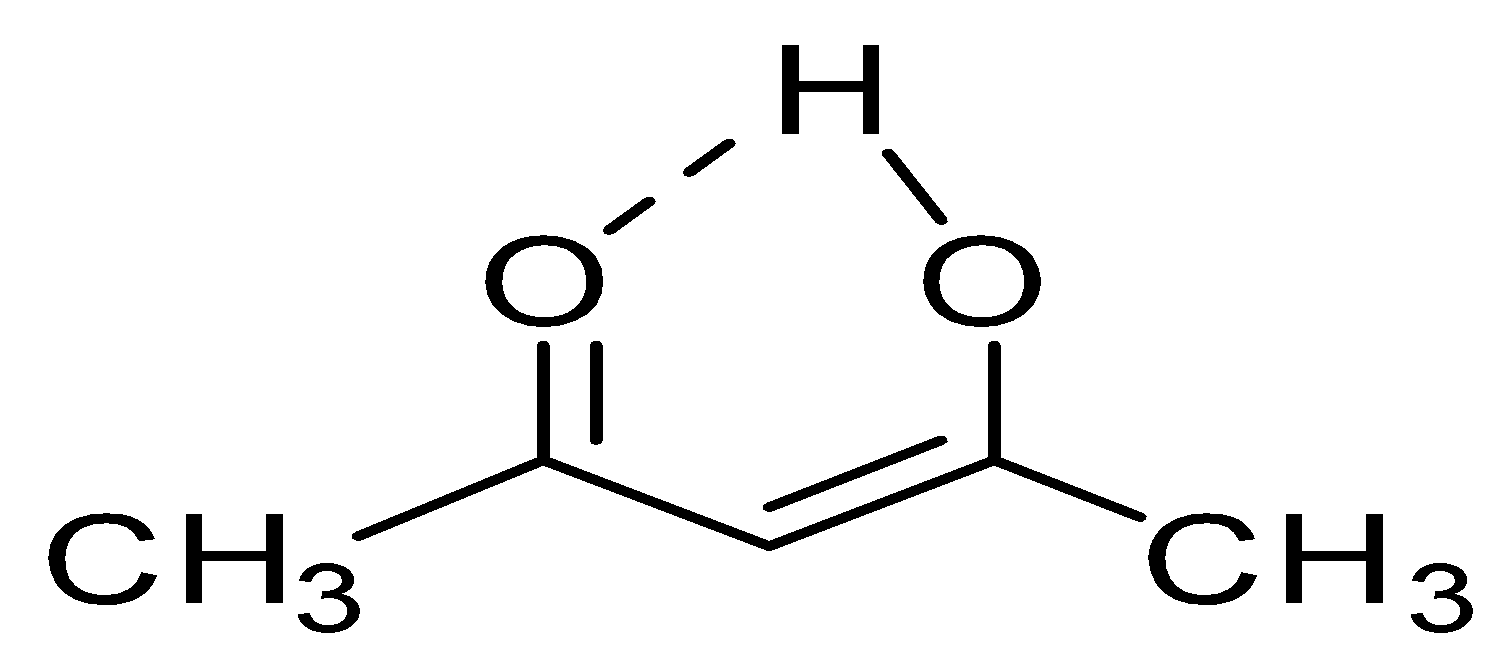
2. Enol content of acetyl acetaldehyde ($C{{H}_{3}}COC{{H}_{2}}CHO$) will be at second number because hydrogen bonding will be there but because of having one less methyl group, it will have less number of alpha hydrogens that’s double bond will be less stable in comparison to acetyl acetone.
Enolic form of acetyl acetaldehyde is shown below:
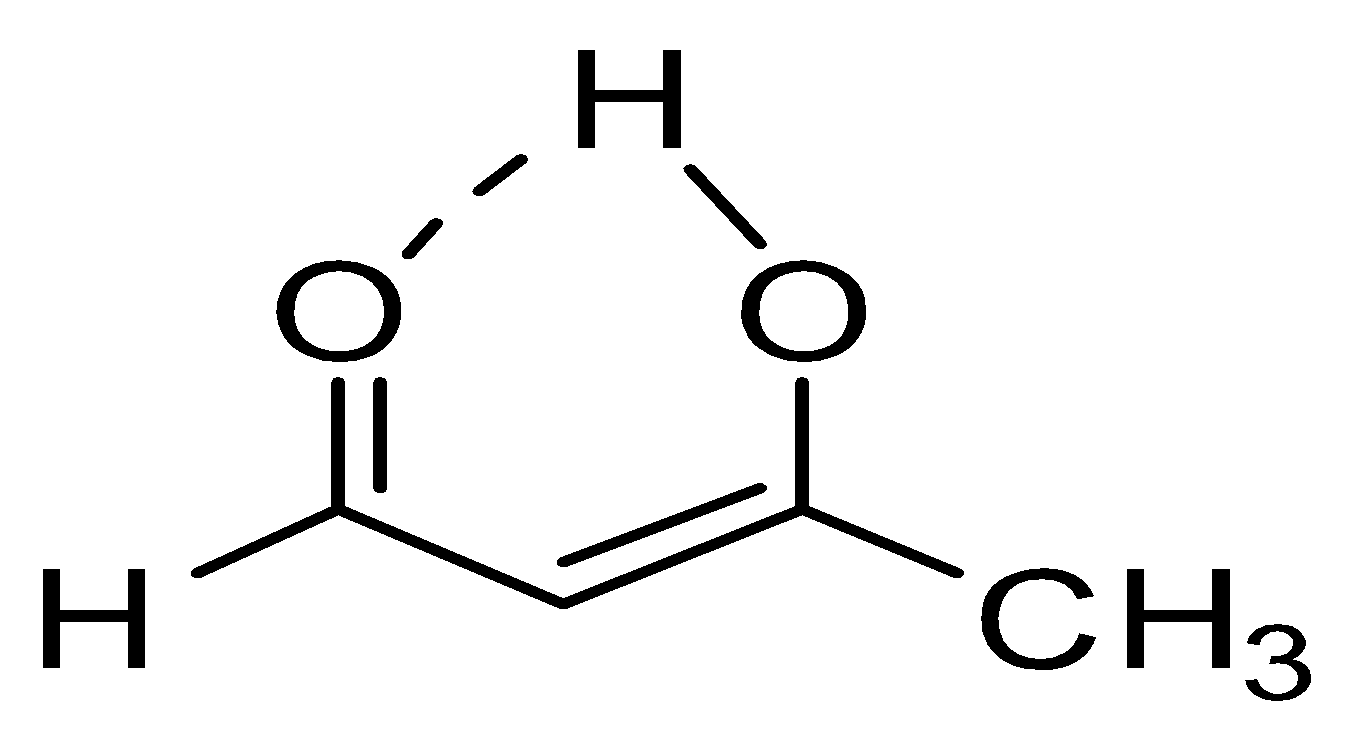
3. At third number acetone ($C{{H}_{3}}COC{{H}_{3}}$) will be there as no hydrogen bonding is present but presence of two methyl groups will make hyperconjugation possible and that will stabilize the double bond little more in comparison to acetaldehyde. So enol content order will be iii < ii < i < iv
So, the correct answer is “Option B”.
Additional information: Enol content of acetaldehyde is zero, it only exists in its keto form and enol content of acetone is also very very low but slightly higher than acetaldehyde.
Note: Tautomers are isomers of a compound which differ only in the position of the protons and electrons. The carbon skeleton of the compound is unchanged. A reaction which involves simple proton transfer in an intramolecular fashion is called a tautomerism.
Complete Solution :
- Among all 4 compounds $C{{H}_{3}}COC{{H}_{2}}COC{{H}_{3}}$ acetyl acetone has highest enol content.
- Here you can observe in the enol form of acetylacetone that hydrogen of OH group is forming a hydrogen bond with oxygen of carbonyl carbon, along with that alpha carbon from double bond has three alpha hydrogens, so this double bond will be stabilized by hyperconjugation.
- So hydrogen bonding and hyperconjugation together are increasing the stability of enol form of acetylacetone and that’s why it has the highest enolic content.
Enolic form of acetylacetone is shown below:

2. Enol content of acetyl acetaldehyde ($C{{H}_{3}}COC{{H}_{2}}CHO$) will be at second number because hydrogen bonding will be there but because of having one less methyl group, it will have less number of alpha hydrogens that’s double bond will be less stable in comparison to acetyl acetone.
Enolic form of acetyl acetaldehyde is shown below:

3. At third number acetone ($C{{H}_{3}}COC{{H}_{3}}$) will be there as no hydrogen bonding is present but presence of two methyl groups will make hyperconjugation possible and that will stabilize the double bond little more in comparison to acetaldehyde. So enol content order will be iii < ii < i < iv
So, the correct answer is “Option B”.
Additional information: Enol content of acetaldehyde is zero, it only exists in its keto form and enol content of acetone is also very very low but slightly higher than acetaldehyde.
Note: Tautomers are isomers of a compound which differ only in the position of the protons and electrons. The carbon skeleton of the compound is unchanged. A reaction which involves simple proton transfer in an intramolecular fashion is called a tautomerism.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




