
Arrange the following in decreasing order of their acidic strengths:
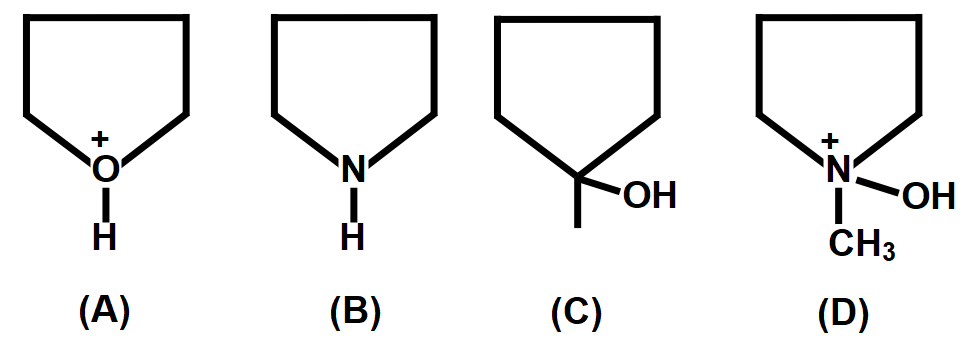
A.$A>C>B>D$
B.$A>D>B>C$
C.$A>D>C>B$
D.None of the above.

Answer
509.1k+ views
Hint: We know that the acid strength of an acid is defined as the efficiency of acid to dissociate into its constituent anion and cation. The acidic strength of acid depends on the inductive effect of the group attached to the substituent compound.
Complete answer:
As we know that the acidic strength depends on the inductive effect. The \[+I\] effect group (electron releasing group) decreases the acidic strength and \[-I\] effect group (electron withdrawing group) increases the acidic strength. The inductive effect is defined as the formation of permanent dipole moments in a molecule which is generated by the unequal sharing of bonding electrons. When an electron withdrawing group and an electron releasing group are attached to the compound, negative and positive charge is incorporated in the compound due to the group added. This results in permanent dipole moments in the molecule resulting in an inductive effect. The inductive effect is divided into \[+I\] effect inductive effect and \[-I\] effect inductive effect based on the group attached to the compound. When an electron releasing group is attached which can donate its electrons then the $+$ inductive effect is formed.
With increase in the number of electrons withdrawing groups, the acid strength increases. With increase in the strength of \[-I\] effect of electron withdrawing groups, the acid strength increases. Compound A is most acidic among the given compounds because the tertiary carbon atom has three strong electrons withdrawing, hydroxyl groups. When this tertiary carbon atom loses an electron, it forms a carbanion in which the negative charge is delocalized through resonance with three carbonyl groups. Greater is the resonance, greater is the stabilization of the carbanion and greater is the acid strength. After compound A, the compound D is most acidic among the given compounds because one of the secondary carbon atoms has two strong electrons withdrawing, carbonyl groups. When this carbon atom loses an electron, it forms a carbanion in which the negative charge is delocalized through resonance with two carbonyl groups. Greater is the resonance, greater is the stabilization of the carbanion and greater is the acid strength. Then lies compound C and B according to their acidic strength.
Therefore, correct answer is option C i.e. the decreasing order of their acidic strengths:
$A>D>C>B.$
Additional information: The Electron withdrawing groups show \[-I\] effect and electron releasing groups show \[+I\] effect. Here, ‘I’ represents an inductive effect. When electron withdrawing groups are present, the acid strength increases and when electron donating groups are present, the acid strength decreases. When an electron withdrawing group is attached which cannot donate the electrons, -Inductive effect is observed. The \[+I\] effect group decreases the acidic strength and \[-I\] effect group increases the acidic strength.
Note:
Remember that the strong acid can easily donate a proton. The carbanion obtained by donation of a proton is stabilized due to inductive and resonance effects. Greater is the stabilization of the carbanion through inductive and resonance effects, greater is the acid strength of the molecule.
Complete answer:
As we know that the acidic strength depends on the inductive effect. The \[+I\] effect group (electron releasing group) decreases the acidic strength and \[-I\] effect group (electron withdrawing group) increases the acidic strength. The inductive effect is defined as the formation of permanent dipole moments in a molecule which is generated by the unequal sharing of bonding electrons. When an electron withdrawing group and an electron releasing group are attached to the compound, negative and positive charge is incorporated in the compound due to the group added. This results in permanent dipole moments in the molecule resulting in an inductive effect. The inductive effect is divided into \[+I\] effect inductive effect and \[-I\] effect inductive effect based on the group attached to the compound. When an electron releasing group is attached which can donate its electrons then the $+$ inductive effect is formed.
With increase in the number of electrons withdrawing groups, the acid strength increases. With increase in the strength of \[-I\] effect of electron withdrawing groups, the acid strength increases. Compound A is most acidic among the given compounds because the tertiary carbon atom has three strong electrons withdrawing, hydroxyl groups. When this tertiary carbon atom loses an electron, it forms a carbanion in which the negative charge is delocalized through resonance with three carbonyl groups. Greater is the resonance, greater is the stabilization of the carbanion and greater is the acid strength. After compound A, the compound D is most acidic among the given compounds because one of the secondary carbon atoms has two strong electrons withdrawing, carbonyl groups. When this carbon atom loses an electron, it forms a carbanion in which the negative charge is delocalized through resonance with two carbonyl groups. Greater is the resonance, greater is the stabilization of the carbanion and greater is the acid strength. Then lies compound C and B according to their acidic strength.
Therefore, correct answer is option C i.e. the decreasing order of their acidic strengths:
$A>D>C>B.$
Additional information: The Electron withdrawing groups show \[-I\] effect and electron releasing groups show \[+I\] effect. Here, ‘I’ represents an inductive effect. When electron withdrawing groups are present, the acid strength increases and when electron donating groups are present, the acid strength decreases. When an electron withdrawing group is attached which cannot donate the electrons, -Inductive effect is observed. The \[+I\] effect group decreases the acidic strength and \[-I\] effect group increases the acidic strength.
Note:
Remember that the strong acid can easily donate a proton. The carbanion obtained by donation of a proton is stabilized due to inductive and resonance effects. Greater is the stabilization of the carbanion through inductive and resonance effects, greater is the acid strength of the molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




