
Arrange the following acids in decreasing order of acidity:
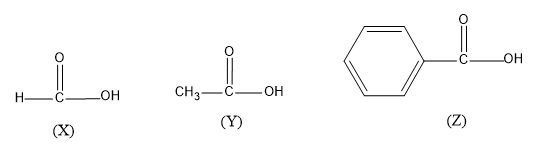
(A) $X\rangle Z\rangle Y$
(B) $X\rangle Y\rangle Z$
(C) $Z\rangle X\rangle Y$
(D) $Z\rangle Y\rangle X$

Answer
561.6k+ views
Hint: Check whether electron withdrawing group or electron donating group is present in the molecule.
- Acidic nature of substance is the ability of the molecule to donate ${{H}^{+}}$ ions during solvation.
Complete step by step answer:
- So in the question three organic molecule structures three given with different groups attached to the carbonyl carbon. All the three molecules have carbonyl carbon in common. Carbonyl carbon is the carbon which possesses a double bond attached to oxygen molecules. And we have to find the most acidic molecule from the options.
- We know that acidity is the tendency of the molecule to furnish ${{H}^{+}}$ ions in the aqueous solution. When the molecule is dissolved in water then the molecule which has a high tendency to donate ${{H}^{+}}$ ions easily are referred as most acidic molecules.
So we have to know from the given molecules which have the ability to furnish protons easily.
- First analyze the three compounds, so we know that as each molecule has a carbonyl group and a –OH group present the molecules given are carboxylic acid. Considering the nature of the groups attached to the functional group it gives an idea about the stability and acidic parameter of the molecules.
- If we consider the structure Z, then a benzene ring is attached to the carbonyl carbon which is resonance stabilized, hence the molecule will be highly stable by the resonance in benzene and the benzene is a very potent electron withdrawing group and it attracts more electrons towards and negative charge will be aggregated, hence molecule can easily donate ${{H}^{+}}$ ions in aqueous solution. So this molecule is more acidic than the others.
Next let’s consider the structure Y, here the carbonyl carbon is attached to a methyl group ($-C{{H}_{3}}$).The methyl group is an electron donating group, the group donates more electrons to carbonyl C which destabilizes the molecule and is hard to furnish ${{H}^{+}}$ ions in aqueous solution.
- Now take the structure of the X molecule where the carbonyl carbon is attached to the H atom which has merely no effect on the acidic parameter. Since there is no electron withdrawing group or electron donating group attached with the carboxylic ,the molecule have a decent stability between the range of structure Z and Y
Hence, the decreasing order of acidity for the given question is,$Z\rangle X\rangle Y$, The correct option is option “C” .
Note: If electron withdrawing groups are attached then they will stabilize the molecule and the acidic character will be more as they attract electrons towards themselves and more negative charge is aggregated which lead to increase in polarity between the bonds and helps in furnishing ${{H}^{+}}$ ions .
Some of the electrons withdrawing groups are: $-X,-CHO,-COR,-COOR$ etc.
- If electron donating groups are attached, they destabilize the molecule and will be less acidic since they donate more electrons towards C attached and make the carbon more negative which makes the ability to donate ${{H}^{+}}$ ions weaker.
Some of the electron donating groups are: $-R,-N{{R}_{2}},-N{{H}_{2}}-OR$ etc.
- Acidic nature of substance is the ability of the molecule to donate ${{H}^{+}}$ ions during solvation.
Complete step by step answer:
- So in the question three organic molecule structures three given with different groups attached to the carbonyl carbon. All the three molecules have carbonyl carbon in common. Carbonyl carbon is the carbon which possesses a double bond attached to oxygen molecules. And we have to find the most acidic molecule from the options.
- We know that acidity is the tendency of the molecule to furnish ${{H}^{+}}$ ions in the aqueous solution. When the molecule is dissolved in water then the molecule which has a high tendency to donate ${{H}^{+}}$ ions easily are referred as most acidic molecules.
So we have to know from the given molecules which have the ability to furnish protons easily.
- First analyze the three compounds, so we know that as each molecule has a carbonyl group and a –OH group present the molecules given are carboxylic acid. Considering the nature of the groups attached to the functional group it gives an idea about the stability and acidic parameter of the molecules.
- If we consider the structure Z, then a benzene ring is attached to the carbonyl carbon which is resonance stabilized, hence the molecule will be highly stable by the resonance in benzene and the benzene is a very potent electron withdrawing group and it attracts more electrons towards and negative charge will be aggregated, hence molecule can easily donate ${{H}^{+}}$ ions in aqueous solution. So this molecule is more acidic than the others.
Next let’s consider the structure Y, here the carbonyl carbon is attached to a methyl group ($-C{{H}_{3}}$).The methyl group is an electron donating group, the group donates more electrons to carbonyl C which destabilizes the molecule and is hard to furnish ${{H}^{+}}$ ions in aqueous solution.
- Now take the structure of the X molecule where the carbonyl carbon is attached to the H atom which has merely no effect on the acidic parameter. Since there is no electron withdrawing group or electron donating group attached with the carboxylic ,the molecule have a decent stability between the range of structure Z and Y
Hence, the decreasing order of acidity for the given question is,$Z\rangle X\rangle Y$, The correct option is option “C” .
Note: If electron withdrawing groups are attached then they will stabilize the molecule and the acidic character will be more as they attract electrons towards themselves and more negative charge is aggregated which lead to increase in polarity between the bonds and helps in furnishing ${{H}^{+}}$ ions .
Some of the electrons withdrawing groups are: $-X,-CHO,-COR,-COOR$ etc.
- If electron donating groups are attached, they destabilize the molecule and will be less acidic since they donate more electrons towards C attached and make the carbon more negative which makes the ability to donate ${{H}^{+}}$ ions weaker.
Some of the electron donating groups are: $-R,-N{{R}_{2}},-N{{H}_{2}}-OR$ etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




