
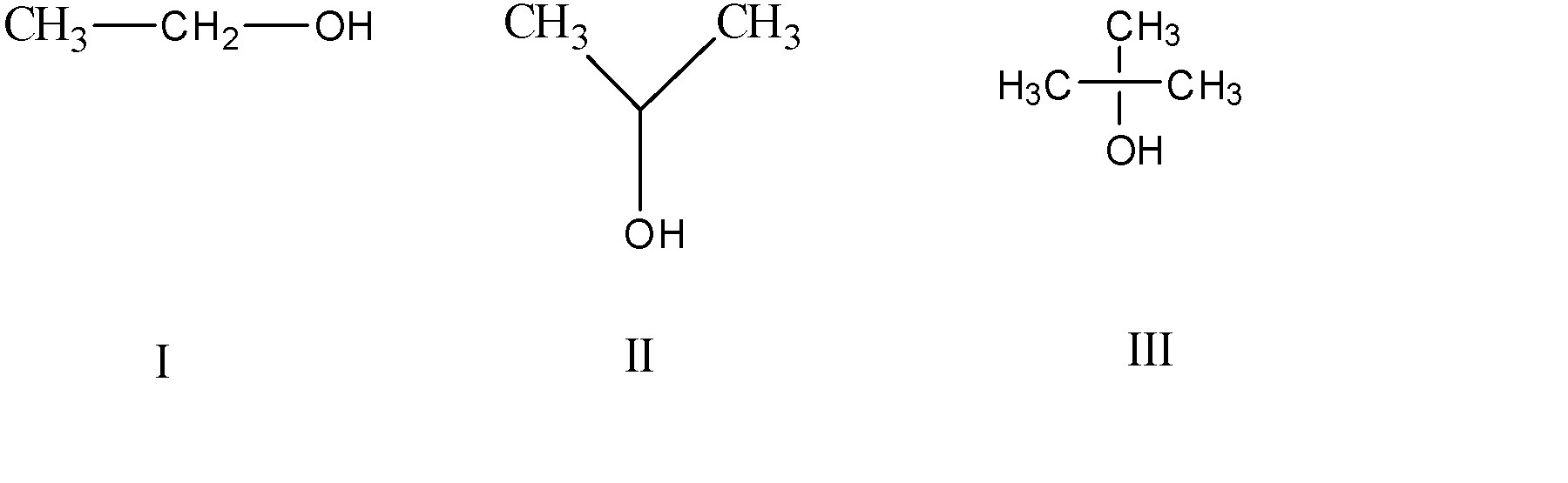
Arrange in increasing order of acidity
A. I > II > III
B. III > II > I
C. I > III > II
D. II > I > III

Answer
574.8k+ views
Hint: In Chemistry, there are three types of substances namely acidic, basic, and neutral. It all is related to the amount of hydrogen ion that the compound can generate. The acidic substance turns moist litmus paper bred and the basic ones turn the litmus paper blue. The neutral compounds don't affect the litmus paper.
Complete step by step answer:
The inductive effect $(I)$ is the effect regarding the transmission of the inequality in the sharing of the electron during the bonding process through the chain of atoms in the molecule.it may be the reason for a permanent dipole in the compound due to this effect.
The electron-withdrawing effect is termed negative ($ - I$) and the electron-donating effect is termed the positive inductive effect ($ + I$).
Acidic strength of the compound is increased due to the presence of the groups showing ($ - I$) effect. The $ - OH$ group present is electrons withdrawing through the inductive effect.
The strongest acid must have the most stable conjugate base. That is why for the alcohol, the acidity trend is $1^\circ > 2^\circ > 3^\circ $ . The methyl groups compensate for the electrons withdrawn by the hydroxyl group. The tertiary carbon compensates the highest for the inductive effect hence nullifying it but the primary carbon is not able to do so hence is the most acidic.
So, the correct answer is Option A.
Note: Though the hydroxyl group is electron-withdrawing by inductive effect, it is electron-donating through the resonance effect.
The mesomeric effect is the property that depends on the substituent group that is attached to the main compound.it is related to the polarity shift that occurs in the molecule due to the presence of the substituent group on the main compound. The interaction of the pi- bonds with the lone pair of the compound or another pi-bond is the action that results in the mesomeric effect.
Complete step by step answer:
The inductive effect $(I)$ is the effect regarding the transmission of the inequality in the sharing of the electron during the bonding process through the chain of atoms in the molecule.it may be the reason for a permanent dipole in the compound due to this effect.
The electron-withdrawing effect is termed negative ($ - I$) and the electron-donating effect is termed the positive inductive effect ($ + I$).
Acidic strength of the compound is increased due to the presence of the groups showing ($ - I$) effect. The $ - OH$ group present is electrons withdrawing through the inductive effect.
The strongest acid must have the most stable conjugate base. That is why for the alcohol, the acidity trend is $1^\circ > 2^\circ > 3^\circ $ . The methyl groups compensate for the electrons withdrawn by the hydroxyl group. The tertiary carbon compensates the highest for the inductive effect hence nullifying it but the primary carbon is not able to do so hence is the most acidic.
So, the correct answer is Option A.
Note: Though the hydroxyl group is electron-withdrawing by inductive effect, it is electron-donating through the resonance effect.
The mesomeric effect is the property that depends on the substituent group that is attached to the main compound.it is related to the polarity shift that occurs in the molecule due to the presence of the substituent group on the main compound. The interaction of the pi- bonds with the lone pair of the compound or another pi-bond is the action that results in the mesomeric effect.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction

State the laws of reflection of light




